- Home
- Cryopreservation Just Got Better with Optibumin® 25
Cryopreservation Just Got Better with Optibumin® 25
Published on 19 February 2025
Increase T Cell Recovery, Reduce DMSO, and Seamlessly Replace Plasma-Derived HSA
Reading time: 6 minutes
Share this article:

Optimizing Cryopreservation with Animal-Origin-Free HSA Solutions
Struggling with cell viability after thawing? You’re not alone. Cryopreservation plays a crucial role in biomanufacturing, especially in developing cell-based therapies, where post-thaw viability can impact everything from functionality to clinical success. Traditionally, dimethyl sulfoxide (DMSO) has been the go-to cryoprotectant, but its toxicity and increasing regulatory scrutiny have scientists searching for safer, more effective alternatives.
At the same time, human serum albumin (HSA) derived from blood has been a key component of cryopreservation media, but batch-to-batch variability, pathogen transmission risks, and supply chain constraints make it less ideal for modern cell therapy workflows.
Now, there’s a better way.
Optibumin® 25 is the first human & animal-origin-free 25% recombinant HSA solution, providing a like-kind replacement for plasma-derived HSA while offering a significant reduction in DMSO concentrations without sacrificing T cell viability and expansion.
Optibumin 25: The Ideal Replacement for Plasma-Derived 25% HSA
Key Benefits of Optibumin 25 in Cryopreservation
Improved T Cell Viability and Expansion Post-Thaw
- T cells cryopreserved with Optibumin 25 in CryoStor® CS10 (10% DMSO) demonstrated up to 2x expansion over 72 hours compared to to less than 1.5x plasma-derived HSA.
- Similar improvements were seen in CryoStor CS5 (5% DMSO), with Optibumin achieving 1.6-fold and 1.25-fold expansions across two donors.
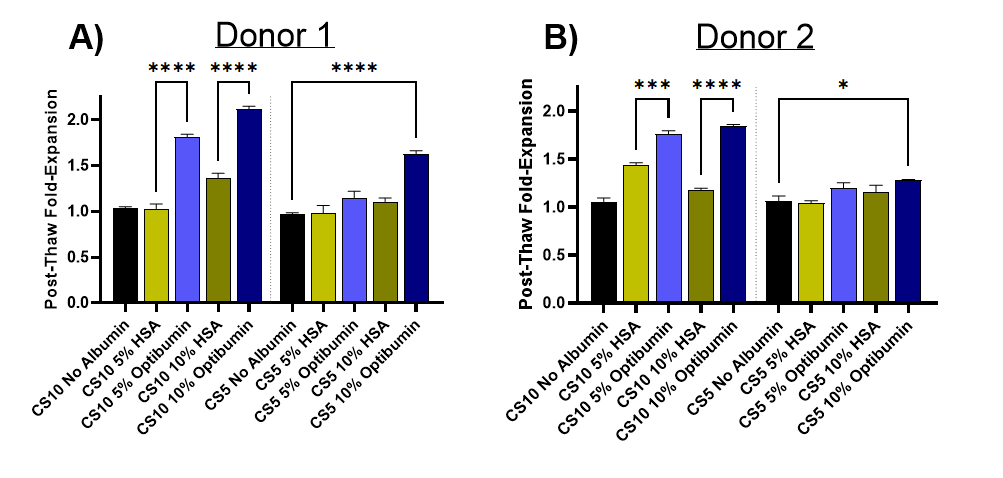
Significant Reduction of DMSO for Safer Final Formulations
- High concentrations of DMSO are associated with toxicity and adverse reactions upon infusion in patients.
- By incorporating 5% or 10% Optibumin 25 into CryoStor formulations, developers can reduce final DMSO concentrations by up to 40%:
- CryoStor CS10 (typically 10% DMSO) → Reduced to 6% with Optibumin
- CryoStor CS5 (typically 5% DMSO) → Reduced to 3% with Optibumin
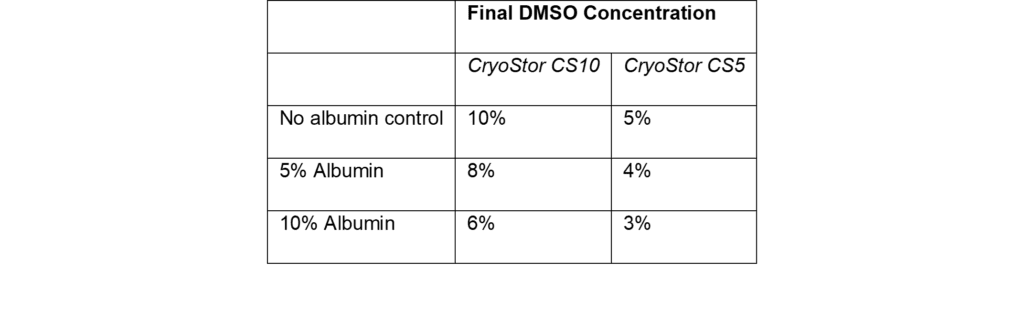
💡 Lower DMSO levels = improved patient safety without compromising T cell viability.
Preservation of Critical Memory T Cell Phenotypes
- Optibumin 25 preserved early memory T cell phenotypes like stem cell memory and central memory (Tscm and Tcm), crucial for CAR-T therapy durability and efficacy, maintaining their proportions 24 and 72 hours post-thaw compared to serum-derived HSA and no albumin controls.
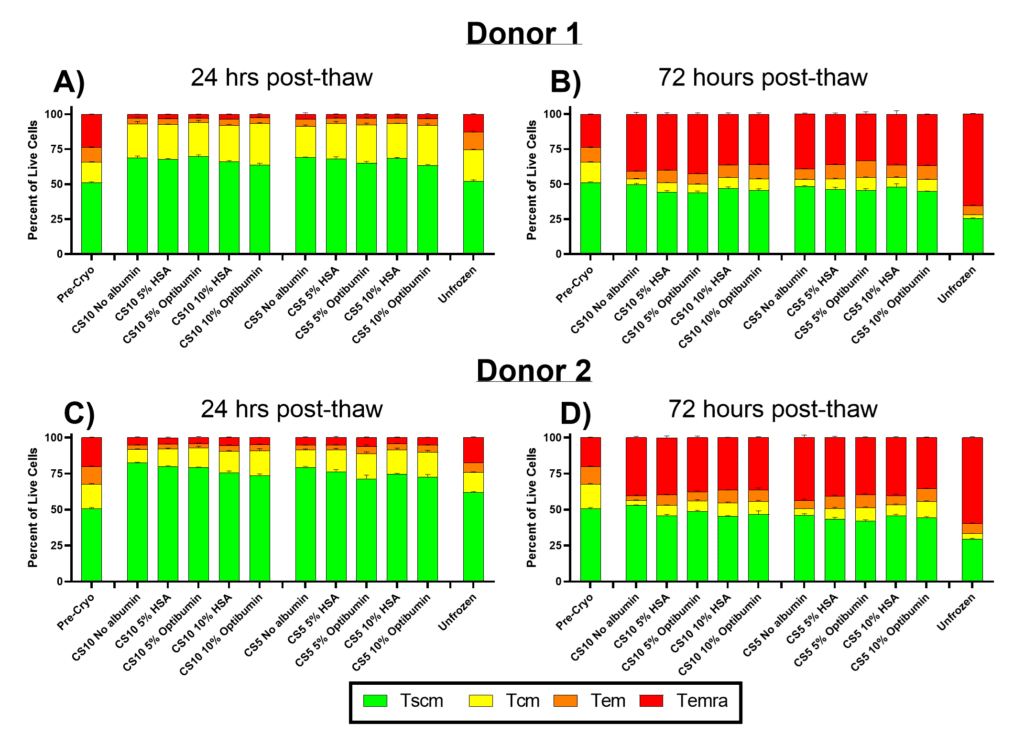
Maintaining CD8+ T Cell Populations
- A balanced CD4/CD8 ratio is essential for CAR-T manufacturing, as higher CD4/CD8 ratios can reduce therapeutic efficacy.
- Optibumin 25 significantly preserved CD8+ cytotoxic T cell populations, which are critical for therapeutic potency, reducing CD8+ cell loss compared to plasma-derived HSA.
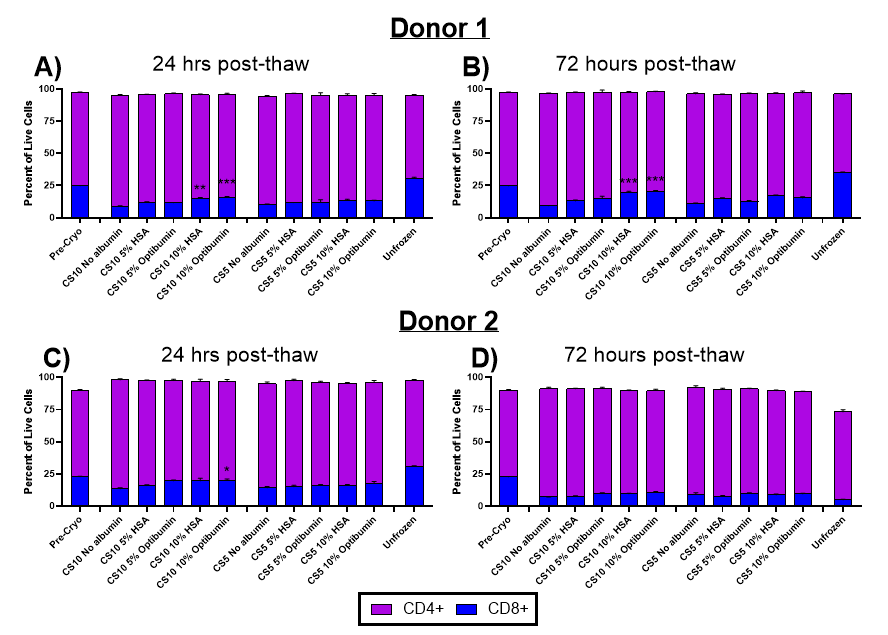
Why Reducing DMSO in Cryopreservation Matters
Lower DMSO Levels Improve Patient Safety
DMSO is not just toxic to cells—it can also cause adverse reactions in patients when infused, including:
- Nausea, vomiting, and abdominal pain.
- Headaches and respiratory distress.
- Severe allergic responses, including hypotension in sensitive individuals.
Regulatory agencies, including the FDA and EMA, strongly encourage lowering DMSO concentrations in cryopreserved cell therapies to mitigate infusion-related toxicities. Optibumin 25 allows developers to reduce DMSO by up to 40%, significantly improving patient safety.
DMSO is Cytotoxic at High Concentrations
While DMSO prevents ice damage, it is also a known cytotoxic agent, particularly at concentrations above 5-10%. Prolonged exposure can:
- Induce apoptosis and oxidative stress in sensitive cell types.
- Reduce post-thaw viability and impair cell proliferation.
- Alter cell function, potentially impacting therapeutic efficacy.
Reducing DMSO concentrations helps enhance cell safety, ensuring higher viability and better therapeutic outcomes.
Regulatory Trends Favor Lower DMSO in Biopharmaceuticals
As cell and gene therapies become more mainstream, regulatory bodies are placing greater scrutiny on cryoprotectant formulations.
- EMA & FDA Guidelines recommend that biopharmaceutical manufacturers limit DMSO exposure in final formulations.
- Commercialized cell therapies (such as CAR-T products) often require extensive DMSO washout procedures, adding cost and complexity to manufacturing.
Reducing DMSO concentrations early in cryopreservation simplifies downstream processing, improves regulatory compliance, and future-proofs cell therapy manufacturing.
Conclusion: Reduce DMSO, Improve Cryopreservation with Optibumin 25
- Significantly reduces DMSO concentrations—by up to 40%.
- Improves T cell viability, expansion, and memory phenotype preservation.
- Seamlessly integrates into CryoStor workflows with no process modifications.
Get the Application Note: Enhanced Cryopreservation of T Cells Using Optibumin®, Recombinant Human Serum Albumin (rHSA)
Ready to Learn More?
Let’s talk! Reach out today to see how Optibumin 25 can help improve your cryopreservation process.
Stay at the Forefront of Innovation
At InVitria, we’re dedicated to pushing the boundaries of biomanufacturing with advanced, recombinant, and chemically defined solutions. Our team is constantly innovating to support the industry’s evolving needs. Sign up for our newsletter to get early access to product updates, expert insights, and exclusive content.
Explore InVitria’s Solutions
- Optibumin® 25 (in bags) – A high-purity recombinant human serum albumin (rHSA) at 25% concentration, designed for seamless integration into CryoStor-based workflows. Optibumin 25 enables:
- Up to 40% DMSO reduction for safer cryopreservation.
- Enhanced T cell recovery and proliferation post-thaw.
- Preservation of critical CAR-T memory phenotypes.
Footnotes
References
- Burnham, R. E., Van Gool, F., Davies, N., & Groves, T. (2021). Human serum albumin and chromatin condensation rescue ex vivo expanded γδ T cells from the effects of cryopreservation. Cryobiology, 99, 78–87. https://doi.org/10.1016/j.cryobiol.2021.01.011
- Duarte, A. C., Costa, M., & Oliveira, P. (2023). Animal-derived products in science and current alternatives. Biomaterials Advances, 151, 213428. https://doi.org/10.1016/j.bioadv.2023.213428
- Galli, E., et al. (2023). The CD4/CD8 ratio of infused CD19-CAR-T is a prognostic factor for efficacy and toxicity. British Journal of Haematology, 203(4), 564–570. https://doi.org/10.1111/bjh.19117
- Li, R. U. I., Huang, J., & Wang, Y. (2019). Preservation of cell-based immunotherapies for clinical trials. Cytotherapy, 21(9), 943–957. https://doi.org/10.1016/j.jcyt.2019.07.004
- Lin, Y., Raje, N. S., Berdeja, J. G., Siegel, D. S., Jagannath, S., Madduri, D., et al. (2023). Idecabtagene vicleucel for relapsed and refractory multiple myeloma: Post hoc 18-month follow-up of a phase 1 trial. Nature Medicine, 29, 2286–2294. https://doi.org/10.1038/s41591-023-02496-0
- Lu, H., Zhang, Y., & Liu, P. (2024). Identifying new safety risk of human serum albumin: A retrospective study of real-world data. Frontiers in Pharmacology, 15, 1319900. https://doi.org/10.3389/fphar.2024.1319900
- Madsen, B. K., Jensen, T., & Hall, S. (2018). Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Research, 7, 654. https://doi.org/10.12688/f1000research.16642.2
- MacLennan, S., & Barbara, J. A. J. (2006). Risks and side effects of therapy with plasma and plasma fractions. Best Practice & Research Clinical Haematology, 19(1), 169–189. https://doi.org/10.1016/j.beha.2005.01.033
- Meyran, D., et al. (2023). TSTEM-like CAR-T cells exhibit improved persistence and tumor control compared with conventional CAR-T cells in preclinical models. Science Translational Medicine, 15(690), eabk1900. https://doi.org/10.1126/scitranslmed.abk1900
- Tian, Y., et al. (2017). Unique phenotypes and clonal expansions of human CD4 effector memory T cells re-expressing CD45RA. Nature Communications, 8(1), 1473. https://doi.org/10.1038/s41467-017-01728-5
- S. Food and Drug Administration. (2024). Considerations for the use of human- and animal-derived materials in the manufacture of cell and gene therapy and tissue-engineered medical products. U.S. Department of Health and Human Services, Center for Biologics Evaluation and Research. https://www.regulations.gov/docket/FDA-2024-D-1244
- Van der Walle, C. F., Shaw, E., & Davidson, L. (2021). Formulation considerations for autologous T cell drug products. Pharmaceutics, 13(8), 1317. https://doi.org/10.3390/pharmaceutics13081317
Legal Notice
Copyright© 2025 InVitria, Inc. All rights reserved.
Reproduction or distribution of any InVitria materials, in whole or in part, is prohibited without prior written consent. All logos, names, designs, and marks displayed, including InVitria®, and the InVitria® brand design, are trademarks or service marks owned or licensed by InVitria, Inc., Kansas, USA, unless otherwise noted. CryoStor® is a registered trademark of BioLife Solutions. For details on InVitria’s registered intellectual property, patents, and additional terms and conditions, please visit www.InVitria.com/terms.