- Home
- Exbumin – Stabilizing Virus with Albumin to Improve Yield Application Note
Exbumin – Stabilizing Virus with Albumin to Improve Yield Application Note
Published on 1 October 2020
Stabilizing Virus with Albumin to Improve Yield
Workflow Summary
Expand virus-producing cells to desired confluency. Remove growth medium and add the desired virus to be expanded (virus or plasmid DNA). Add Exbumin (Product Number: 777HSA097S) to the expression media and allow virus to propagate. Harvest culture and determine virus titer by preferred method.
Here, a general protocol is described for the addition of recombinant albumin, in the form of the product Exbumin, to virus production systems to stabilize infectious particles and thereby maximize viral yield.
Introduction
Virus production is an important application of cell culture systems, both for vaccine manufacturing as well as for gene therapy. During the cell culture process, the infectious particles produced are subjected to several physical stresses inherent to the cell culture system that may cause inactivation and reduce the overall productivity of the culture [1]. Specific culture parameters can be modified in order to prevent virus inactivation. For example, measles virus production has been shown to be enhanced in cell cultures by concentrating serum in the medium [2]. Likewise, a more basic culture pH has been shown to enhance harvest yields of Japanese B encephalitis virus [1]. The addition of albumin has also been shown to enhance virus yields in vitro [3]. However, the use of serum-derived proteins can drive heterogeneity of culture performance, increase the risk of introducing adventitious agents into the production system, and potentially jeopardize the availability of vaccines due to variable supply [4,5]. For this reason, development of recombinant albumin that can provide consistent stabilization is desirable. Indeed, there is evidence that recombinant albumin is beneficial for stability during lyophilization of the attenuated dengue type 2 (DEN-2), strain PDK-53 (DEN-2 PDK) vaccine [6]. Further, recombinant albumin has been utilized to stabilize viral vaccine components for human clinical use in vaccines for mumps, measles, rubella, dengue, West Nile and yellow fever, among others [5].
Materials
Virus-Producing Cell Line(s)
- HEK293
- HEK293t
- VERO
- MDCK
- MRC-5
- A549
- Wi38
- CEF
- Sf9
Virus Expression Media
- OptiVERO – blood-free and defined complete media; Product Number: 777OCM090
- Expi293 – Life Technologies
- VP-SFM – Life Technologies
- Basal Classical Medium + Fetal Bovine Serum
Exbumin
- Exbumin – Recombinant human serum albumin; Product Number: 777HSA097S
Protocol
Exbumin Stock Preparation
- To prepare concentrated liquid stocks of Exbumin, gently dissolve Exbumin powder in cell culture grade DPBS, PBS, or basal media to between 10mg/mL to 20mg/mL.
- Use gentle mixing or inversion to minimize the formation of bubbles. Allow Exbumin to dissolve for at least 1 hour at room temperature or overnight at 4°C.
- Liquid stocks should be sterile-filtered prior to use. A pre-filtration step with a 0.8µm filter is recommended prior to a final sterile filtration step with a 0.2µm filter. Liquid solutions should be stored at 4°C in the dark.
Exbumin Titration in Virus Expansion Media
- As a general guideline, concentrations of Exbumin between 500-1500 mg/L would be enough for virus stabilization during the virus production phase of the culture. However, different virus types and cellular substrates will have optimal inclusion levels of albumin to enhance virus titer and not have detrimental effects to viable cell density.
- Virus producing cells should be grown to the desired confluence in the expansion media of choice. For information about serum-free expansion of cell cultures for virus production, see our application notes:
- Expansion of VERO Cells Without Serum in Chemically Defined Cell Culture Media
- Virus Production Using VERO Cells in Flasks Without Serum in Chemically Defined Cell Culture Media
- Virus Production Using VERO Cells with Microcarriers Without Serum in Chemically Defined Cell Culture Media
- Once the cells have reached the desired confluency, initiate virus production by transfecting with plasmid DNA or infection by the infectious virus to be expanded (See app note OptiVERO 2D virus expansion).
- Once the infection/transfection media has been removed, exchange the media for virus production media of choice containing 500-1500 mg/L Exbumin.
- Perform the virus production run.
- Quantify virus production by preferred method.
Results and Discussion
Figure 1 demonstrates the results after the addition of Exbumin to virus-producing cultures. As expected, Exbumin enhanced the production of an influenza virus over unsupplemented cultures. Thus, Exbumin recombinant albumin is an attractive and cost-effective alternative for virus stabilization compared to using human serum-derived albumin.
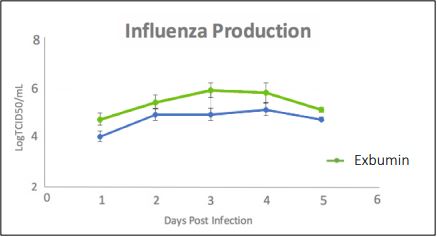
Figure 1. Enhanced virus production in cell cultures supplemented with Exbumin. VERO cells were allowed to adhere on microcarrier beads and expanded in spinner flasks. Cells were subsequently infected with an influenza virus and the culture medium was partially exchanged for the same formulation with and without Exbumin. Culture samples were obtained every day and the pH of the cultures were maintained between 6.8 and 7.2 by the addition of NaOH. Agitation of the cultures was kept constant throughout the process. The effect of Exbumin addition to the cultures was beneficial to viral titers.
For additional product or technical information, please contact us at:
2718 Industrial Drive Phone: 1.800.916.8311
Junction City, KS 66441 Email: Info@InVitria.com
The following content is gated. Please, subscribe to open access to it.
Footnotes
- Scott JV, Choppin PW. Enhanced yields of measles virus from cultured cells. J Virol Methods. 1982;5(3-4):173-179.
- Darwish MA, Hammon WM. Studies on Japanese B encephalitis virus vaccines from tissue culture. VI. Development of a hamster kidney tissue culture inactivated vaccine for man. (1). Obtaining maximum titers of virus using an attenuated strain of OCT-541. J Immunol. 1966;96(4):691-698.
- Abe K, Ikeda M, Ariumi Y, Dansako H, Kato N. Serum-free cell culture system supplemented with lipid-rich albumin for hepatitis C virus (strain O of genotype 1b) replication. Virus Res. 2007;125(2):162-168.
- Price PJ, Evege EK. Serum-free medium without animal components for virus production. Focus. 1997;19(3):67-69.
- Wiedmann RT, Reisinger KS, Hartzel J et al. M-M-R(®)II manufactured using recombinant human albumin (rHA) and M-M-R(®)II manufactured using human serum albumin (HSA) exhibit similar safety and immunogenicity profiles when administered as a 2-dose regimen to healthy children. Vaccine. 2015;33(18):2132-2140.
- Wiggan O, Livengood JA, Silengo SJ et al. Novel formulations enhance the thermal stability of live-attenuated flavivirus vaccines. Vaccine. 2011;29(43):7456-7462.