- Home
- T Cell Cryopreservation Using Exbumin®, Recombinant Human Serum Albumin, and Reduced DMSO
T Cell Cryopreservation Using Exbumin®, Recombinant Human Serum Albumin, and Reduced DMSO
Published on 4 November 2024
Application Note
Andrew Hamann, PhD, Product Applications Scientist, InVitria, Inc.
Key Points
- Exbumin®, recombinant human serum albumin (rHSA), demonstrated superior T cell viability, health, and proliferation post-cryopreservation compared to clinical HSA and a 10% DMSO solution. Notably, this was achieved with only 2.5% DMSO, demonstrating Exbumin’s ability to reduce DMSO concentrations without compromising cell health.
- Exbumin is an approved excipient for therapeutic injection and offers a superior alternative to blood-derived albumin, reducing the risk of pathogen transmission while ensuring consistent quality for cell therapy manufacturing.
Introduction
Cryopreservation is a critical step in the manufacturing of T cell-based therapies, which has significant impact on cell health and functionality post-thaw. Optimizing cryopreservation protocols is essential for maintaining T cell potency and improving clinical outcomes. Human serum albumin has been shown to enhance cell health and functionality after thawing (Burnham, 2021) and is included in several on-market cell therapies (Walle, 2021). However, the use of human blood-derived albumin products in cryopreservation formulations presents potential risks, including the possibility of pathogen transmission (MacLennan & Barbara, 2006) or adverse reactions (Lu, 2024). Regulatory agencies encourage pharmaceutical companies to use animal-origin-free materials in therapeutic production (U.S. FDA, 2024).
InVitria’s Exbumin is specifically designed to address key challenges in cell therapy manufacturing. Exbumin provides several crucial advantages:
Safety and Consistency
Exbumin is produced using a plant-based expression system, eliminating the potential risk of contamination from adventitious infectious agents associated with blood-derived albumin. This animal-origin-free production ensures reproducibility and enhances safety for clinical applications. Approved by major regulatory agencies as an excipient in injectable therapeutics, Exbumin has been used in hundreds of thousands of injections worldwide. The use of animal-free alternatives, like Exbumin, plays a critical role in reducing variability and contamination risks in cell culture and therapy applications, as extensively documented (Duarte, 2023).
Regulatory Compliance and Supply Chain Stability
Exbumin is manufactured under cGMP conditions in an ISO 9001:2015-certified facility, meeting stringent quality standards for clinical-grade materials. InVitria’s vertically integrated supply chain ensures a reliable source of high-quality rHSA, essential for uninterrupted cell therapy production. Additionally, the plant-based expression system used by InVitria, provides scalability, sustainability, and cost-effectiveness, offering clear advantages over other expression systems.
In this application note, we present cryopreservation results using Exbumin for activated and expanded T cells, which are relevant to CAR-T cell therapy manufacturing. Our findings demonstrate Exbumin’s efficacy in maintaining T cell health and proliferation post-thaw, offering a robust solution for pharmaceutical and biotech companies developing next-generation cell therapies.
Results and Discussion
Exbumin rHSA Maintains Post-Thaw T Cell Health with Reduced DMSO
The same number and density of cells were used in all cryopreservation formulations, and equal volumes of thawed cells were plated for analysis, ensuring direct comparison between conditions. Total cell recovery and post-thaw viability were comparable across all conditions. However, we focused on assessing the number of healthy cells (viable and non-apoptotic), as apoptotic cells, even if initially viable, are unlikely to remain effective in cell therapy applications.
In Figure 1, we assessed the number of healthy T cells from three different donors immediately after thawing, following cryopreservation in a formulation containing 2.5% DMSO and either 5% or 10% albumin (Exbumin or a clinical HSA product). The results demonstrated that Exbumin formulations consistently recovered comparable or greater numbers of healthy cells compared to clinical HSA across all donors.
Notably, Exbumin formulations containing 2.5% DMSO yielded recoveries of healthy cells similar to those obtained with a standard cryopreservation solution containing 10% DMSO concentrations. Reducing DMSO, a known contributor to adverse reactions in cell therapies (Madsen, 2018), is crucial for improving the safety profile of cryopreserved T cell products. These findings emphasize the value of Exbumin as a key component in optimizing cryopreservation formulations for clinical applications.

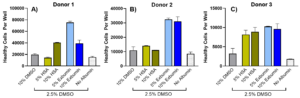
Exbumin rHSA Drives Superior T Cell Growth Post-Thaw:
In Figure 2, the total number of healthy human T cells per well was assessed after three days of culture growth following cryopreservation. Across all three donors, formulations containing Exbumin resulted in significantly higher numbers of healthy T cells (viable and non-apoptotic) compared to a standard cryopreservation solution containing 10% DMSO, with 2- to 3-times more healthy cells observed. Notably, this superior performance was achieved using only 2.5% DMSO in the Exbumin formulations, further underscoring Exbumin’s ability to reduce DMSO concentrations without compromising cell health.
In both Donor 1 and Donor 2, at least one Exbumin formulation significantly outperformed the clinical HSA, demonstrating superior post-thaw cell recovery and growth. For Donor 3, Exbumin still showed a slight improvement over HSA. These results position Exbumin as a highly effective rHSA option for enhancing healthy post-thaw T cell recovery, making it a valuable tool for optimizing cryopreservation protocols for clinical applications. The increased cell proliferation observed with Exbumin formulations may translate to improved outcomes in cell therapy manufacturing, ultimately leading to more effective therapies for patients.
Conclusion
The results presented in this application note demonstrate the efficacy of Exbumin in maintaining T cell viability and promoting healthy cell proliferation post-cryopreservation. Exbumin formulations consistently outperformed or matched clinical HSA and the standard cryopreservation solution containing 10% DMSO in both immediate post-thaw cell recovery and subsequent expansion in culture. Notably, Exbumin achieved these results while utilizing significantly lower DMSO concentrations (2.5% vs. 10% in the standard cryopreservation solution), which is crucial for minimizing potential adverse effects during infusion in clinical applications.
The performance of Exbumin in these studies underscores its value as a superior alternative to blood-derived albumin, optimizing cryopreservation protocols for T cell-based therapies while offering an enhanced safety profile, consistency, and scalability for use in cell therapy manufacturing.
Materials and Methods
Cell Culture and Activation:
Primary human T cells were obtained from three healthy donors and prepared according to protocols designed to mimic workflows used in clinical cell therapy administration. T cells were activated for 3 days using anti-CD3 and anti-CD28 T cell activators, followed by expansion for an additional 3 days. The initial concentration of 2 × 10⁵ cells/mL was increased to 1 × 10⁶ cells/mL by the time of cryopreservation.
Cryopreservation:
T cells were cryopreserved using base cryopreservation solutions composed of 75% Plasma-Lyte and 25% of a standard cryopreservation solution containing 10% DMSO. The formulations were further adjusted by titrating additional solutions containing varying concentrations of DMSO and human serum albumins (HSAs), including Exbumin reconstituted as described in our previous Exbumin – Reconstitution Application Note. T cells were cryopreserved at a final concentration of 5 × 10⁶ cells/mL using a common freezing device (Mr. Frosty™). The freezing process was conducted gradually to promote optimal ice crystal formation and minimize cellular damage. After freezing, the cells were transferred to liquid nitrogen storage until thawing.
Thawing and Assessments:
To simulate typical cell therapy processing before administration (Li, 2019), cryovials were thawed, and the cells were incubated at room temperature for 1 hour before being plated in growth medium. Equal volumes of the thawed cells in their respective cryopreservation formulations were seeded into well plates for direct comparison of conditions. Post-thaw cell viability, apoptosis, and proliferation were assessed over a 3-day period. Viability was determined using live-cell imaging techniques and specific staining protocols to differentiate between viable, apoptotic, and non-apoptotic cells.
The following content is gated. Please, subscribe to open access to it.
Footnotes
References
-
- Burnham, R. E., Van Gool, F., Davies, N., & Groves, T. (2021). Human serum albumin and chromatin condensation rescue ex vivo expanded γδ T cells from the effects of cryopreservation. Cryobiology, 99, 78–87. DOI: 1016/j.cryobiol.2021.01.011
- van der Walle, C. F., Shaw, E., & Davidson, L. (2021). Formulation considerations for autologous T cell drug products. Pharmaceutics, 13(8), 1317. doi: 3390/pharmaceutics13081317
- MacLennan, S., & Barbara, J. A. (2006). Risks and side effects of therapy with plasma and plasma fractions. Best Practice & Research Clinical Haematology, 19(1), 169–189. https://doi.org/10.1016/j.beha.2005.01.033
- Lu, H., Zhang, Y., & Liu, P. (2024). Identifying new safety risks of human serum albumin: A retrospective study of real-world data. Frontiers in Pharmacology, 15, 1319900. https://doi.org/10.3389/fphar.2024.1319900
- S. Food and Drug Administration. (2024). Considerations for the use of human- and animal-derived materials in the manufacture of cell and gene therapy and tissue-engineered medical products. U.S. Department of Health and Human Services, Center for Biologics Evaluation and Research.
- Duarte, A. C., Costa, M., & Oliveira, P. (2023). Animal-derived products in science and current alternatives. Biomaterials Advances, 151, 213428. https://doi.org/10.1016/j.bioadv.2023.213428
- Madsen, B. K., Jensen, T., & Hall, S. (2018). Adverse reactions of dimethyl sulfoxide in humans: A systematic review. F1000Research, 7, 654. doi: 12688/f1000research.16642.2
- Li, R. U. I., Huang, J., & Wang, Y. (2019). Preservation of cell-based immunotherapies for clinical trials. Cytotherapy, 21(9), 943–957. https://doi.org/10.1016/j.jcyt.2019.07.004
Copyright © 2024 InVitria, Inc. All rights reserved.
Reproduction or distribution of any InVitria materials, in whole or in part, is prohibited without prior written consent. All logos, names, designs, and marks displayed, including InVitria®, the InVitria® brand design and Exbumin®, are trademarks or service marks owned or licensed by InVitria, Inc., Kansas, USA, unless otherwise noted. For details on InVitria’s registered intellectual property, patents, and additional terms and conditions, please visit www.InVitria.com/terms.