- Home
- Expansion of Adherent HEK293T Cells in Flasks in Chemically Defined Cell Culture Media
Expansion of Adherent HEK293T Cells in Flasks in Chemically Defined Cell Culture Media
Published on 1 November 2019
Optipeak HEK293t - Expansion of HEK293T Cells
Atherly Pennybaker, Media Formulation and Product Applications Specialist, Invitria
Workflow Summary
Combine supplements of OptiPEAK HEK293T media. Generate stock solutions and prepare reagents. Adapt HEK293t cells in log phase growth from serum to OptiPEAK HEK293T chemically defined, serum free media. Subculture HEK293t cells every 2-3 days in T-75 flasks, calculating doubling time and population doublings to determine cumulative expansion results.
Introduction:
For many years, HEK293 cells have been widely accepted in the biotechnology industry due to their remarkable susceptibility for transfection and production of clinically relevant viruses or therapeutic proteins. As the growing field of gene therapy has continued to show promise in the clinic, the demand for a scalable vector production platform with HEK293t has increased as well. These cells are the current workhorse in the evolving field of gene therapy because of their ability to achieve up to one log higher viral titer compared to cultures of HEK293 in suspension. Traditionally, these cells are expanded and subsequently transfected in media supplemented with fetal bovine serum (FBS); however at clinical manufacturing scale, the global supply of FBS cannot support the high volume demand and the lack of lot-to-lot consistency of serum or blood derived proteins encourages the use of chemically defined media. For this reason, InVitria developed OptiPEAK HEK293T, a chemically defined, serum free media to expand HEK293t cells from seed train to full scale manufacturing for gene therapies in active development today. As shown below, performance of this media allows for a switch from serum-based or blood component containing media to a chemically defined alternative without sacrificing performance. [1,2]
Materials:
- Growth Media Preparation
- OptiPEAK HEK293T Components: Base Media, 25X Protein Supplement
- Penicillin-Streptomycin (Life Technologies 15140122)
- Reagent Preparation
- Soybean Trypsin Inhibitor (Life Technologies 17075-029)
- Ethylenediaminetetraacetic Acid (EDTA) (Sigma Aldrich E6758)
- Dulbecco’s Phosphate Buffered Solution (DPBS)
- TrypLE (Life Technologies A1217702)
- 22 um PES Syringe Filter
- 250 mL Sterile Media Bottle
- Cell Passage
- CellBIND T-75 Flask (Corning 430641U)
- 50 mL Conical Tubes
- Class A2 Biological Safety Cabinet
- Centrifuge
- Cell counter or hemocytometer
- 10% CO2 Incubator at 37 degrees Celsius
Protocol:
Growth Media Preparation
- Each OptiPEAK HEK293T media kit consists of a Base Media and a 25x Protein Supplement. Thaw the frozen protein supplement at 37⁰C until partially thawed and complete the thawing process at room temperature.
- Once the protein supplement is thawed completely in the absence of visible precipitates, gently pipette up and down with a 10 mL pipette. Do not vortex.
- Aseptically add the Protein Supplement directly to the Base Media. If desired, add antibiotics after the protein supplement has been added to 10 U/mL or 0.1x-0.5x final concentration. Do not add to a final concentration of 1000 U/mL..
Reagent Preparation
1x TrypLE + 1 mM EDTA
- To a sterile 250 mL media bottle, aseptically add 222.5 mL of DPBS followed by 25 mL of 10x TrypLE and 2.5 mL of 100 mM EDTA.
- Store the solution at 2-8⁰C up to 6 months. This solution can be repeatedly heated and cooled without affecting the activity of the enzyme.
HEK293t Culture Adaptation to OptiPEAK HEK293T Medium
- HEK293T cells should be highly viable and in log phase growth with a confluence no greater than 80% at the time of adaptation.
- Cells should be at least 3 passages from cryopreservation but no more than 10 passages at the time of adaptation.
- An incubator should be calibrated to achieve an internal CO2 level of 10%.
- On the day of culture initiation, add 0.21 mL/cm² (16 mL per T-75) of cold OptiPEAK HEK293T to a CellBIND flask and allow the media to equilibrate for at least 1 hour in standard growth conditions prior to seeding.
- Prewarm the 1x TrypLE + 1 mM EDTA and 1x STI in an incubator or 37⁰C water bath.
- When the reagents have come up to temperature, wash the HEK293T monolayer three times with 0.2 mL/cm2 (15 mL per T-75) DPBS to remove all traces of serum.
- Add 0.04 mL/cm2 (3 mL per T-75) 1x TrypLE + 1 mM EDTA and allow for the cells to come off the growth surface. After cell detachment is observed, add 3 mL of the 1x STI solution and collect the cell suspension into a 50 mL tube.
- Wash the T flask with 22 mL DPBS to collect any residual cells and combine with the 6 mL in the 50 mL tube.
- Pellet the cells by centrifuging at 1200 RPM for 7 minutes. Aspirate the supernatant and resuspend in 5 mL DPBS and obtain a cell count.
- Seed the appropriate volume to achieve a 1-2e4 cell/cm2 seed and subculture in 2-3 days, respectively.
HEK293t 2D Subculture
- When cells are ready for subculture, add 0.53 mL/cm² (40 mL per T-75) of cold OptiPEAK HEK293T in a CellBIND T-Flask and place the flask in the 37⁰C incubator adjusted to 10% CO2 for at least 1 hour prior to starting the passage.
- While the media is equilibrating, place the 1x TrypLE + 1 mM EDTA and 1x STI at 37⁰C and allow to come up to temperature.
- Gently aspirate the spent media from the HEK293T monolayer and add 0.04 mL/cm2 (3 mL per T-75) 1x TrypLE + 1 mM EDTA and allow for the cells to come off the growth surface. NOTE: Do not wash the HEK293t cells with DPBS prior to trypsinization as excessive cell loss will occur.
- Add 0.04 mL/cm2 (3 mL per T-75) of 1x STI once complete detachment is observed.
- From the new flasks, remove 0.08 mL/cm2 (6 mL per T-75) of prewarmed media and add to the cell suspension. Transfer the cell suspension to at 50 mL tube. NOTE: Cells cultured in the SFM will come off the growth surface as sheets. It is extremely critical to break the cell clumps up by pipetting the cells up and down 1-2 times at the time of collection to ensure that the cells are passaged as single cells and not as clumps.
- Wash the T-Flask with an additional 0.16 mL/cm2 (12 mL per T-75 flask) of fresh media to collect any residual cells.
- Pellet the cells by centrifuging at 1200 RPM for 7 minutes. Aspirate the supernatant and resuspend the cell pellet in 5 mL prewarmed growth media.
- Seed the appropriate volume to achieve a 1-2e4 cells/cm2. Cells seeded at 1e4 cells/cm2 will be ready for subculture in 3 days while 2e4 cells/cm2 should be passaged every 2 days. Do not grow cells to high confluencies as doubling times will be affected in the subsequent passages. Expected results are shown below.
Results:
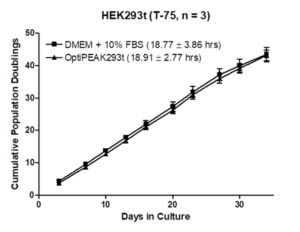
HEK293T cells were adapted to OptiPEAK HEK293T and subsequently expanded for 10 passages and compared to DMEM + 10% FBS. Cells were seeded at 1e4 cells/cm2 and subcultured for 2-3 days at each passage. Each passage consisted of 3 CellBIND T-Flasks for each media. Data is presented below in Figure 1 as cumulative population doublings. The average doubling time was calculated to be 18.91 ± 2.77 hours for OptiPEAK HEK293T and 18.77 ± 3.86 hours for DMEM + 10% FBS. From these results, it can be concluded that OptiPEAK HEK293T exhibits equivalent cell culture performance compared to DMEM + 10% FBS. This makes OptiPEAK HEK293T a superior alternative to the use of undefined components or serum for the expansion of adherent HEK293t cells.
The following content is gated. Please, subscribe to open access to it.
Footnotes
- K. Corsi, F. Chellat, L. Yahia, and J. C. Fernandes, “Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan-DNA nanoparticles,” Biomaterials, vol. 24, no. 7, pp. 1255–1264, Mar. 2003.
- “HEK293T Cell Line – an overview | ScienceDirect Topics.” [Online]. Available: https://www.sciencedirect.com/topics/immunology-and-microbiology/hek293t-cell-line. [Accessed: 06-Sep-2019].