- Home
- Universal Vaccine Against Seasonal Influenza Steps Closer to Reality with the Results of FluGen Phase 2 Clinical Trial
Universal Vaccine Against Seasonal Influenza Steps Closer to Reality with the Results of FluGen Phase 2 Clinical Trial
Published on 19 February 2019
Jeanne McAdara Ph.D.
On Tuesday, the vaccine company FluGen announced promising preliminary results from a placebo-controlled, Phase 2 clinical trial of their investigational intranasal influenza vaccine, M2SR, in 99 healthy adults challenged with the A/Belgium/4217/2015, H3N2 strain.
Worldwide, seasonal influenza causes 3 to 5 million severe infections every year and 250,000 to 500,000 influenza-related deaths. The virus is easily transmissible and notoriously difficult to vaccinate against due to its ability to change its outer hemagglutinin coat through genetic drift and recombination of different variants. Given the speed with which these adaptations take place and the limitations of current technology, a new vaccine must be formulated every year. The three to four component antigens are selected in what is essentially a race against time, based on epidemiologists’ best estimates about which strains and variants will be most prevalent in the coming flu season. Further complicating the process, the biomanufacture of influenza vaccines, carried out in chicken eggs, is time-consuming and there is little opportunity to correct errors if the antigens selected turn out to be mismatched.
Single-Replication Virus Elicits a Robust Host Immune Response
Against this background, FluGen has proposed a novel approach to immunizing people that, if it works, will result in the first “universal” influenza vaccine. Instead of producing a variably efficacious, trivalent (3-antigen) or quadrivalent (4-antigen) injectable vaccine from eggs, FluGen has developed a genetically altered strain of influenza virus that can infect human cells and express viral RNA and proteins, but that is unable to build a second generation of infectious flu particles. Without these particles, no viable virus is produced to spread within the host or be transmitted to new hosts, interrupting the disease cycle. Moreover, the presence of viral RNA and other proteins stimulates a comprehensive host immune response including innate, humoral (B-cell and antibody), and cellular (T-cell) responses—a reaction that could hypothetically result in durable protection against a broader array of influenza virus strains compared to antigen challenge alone.
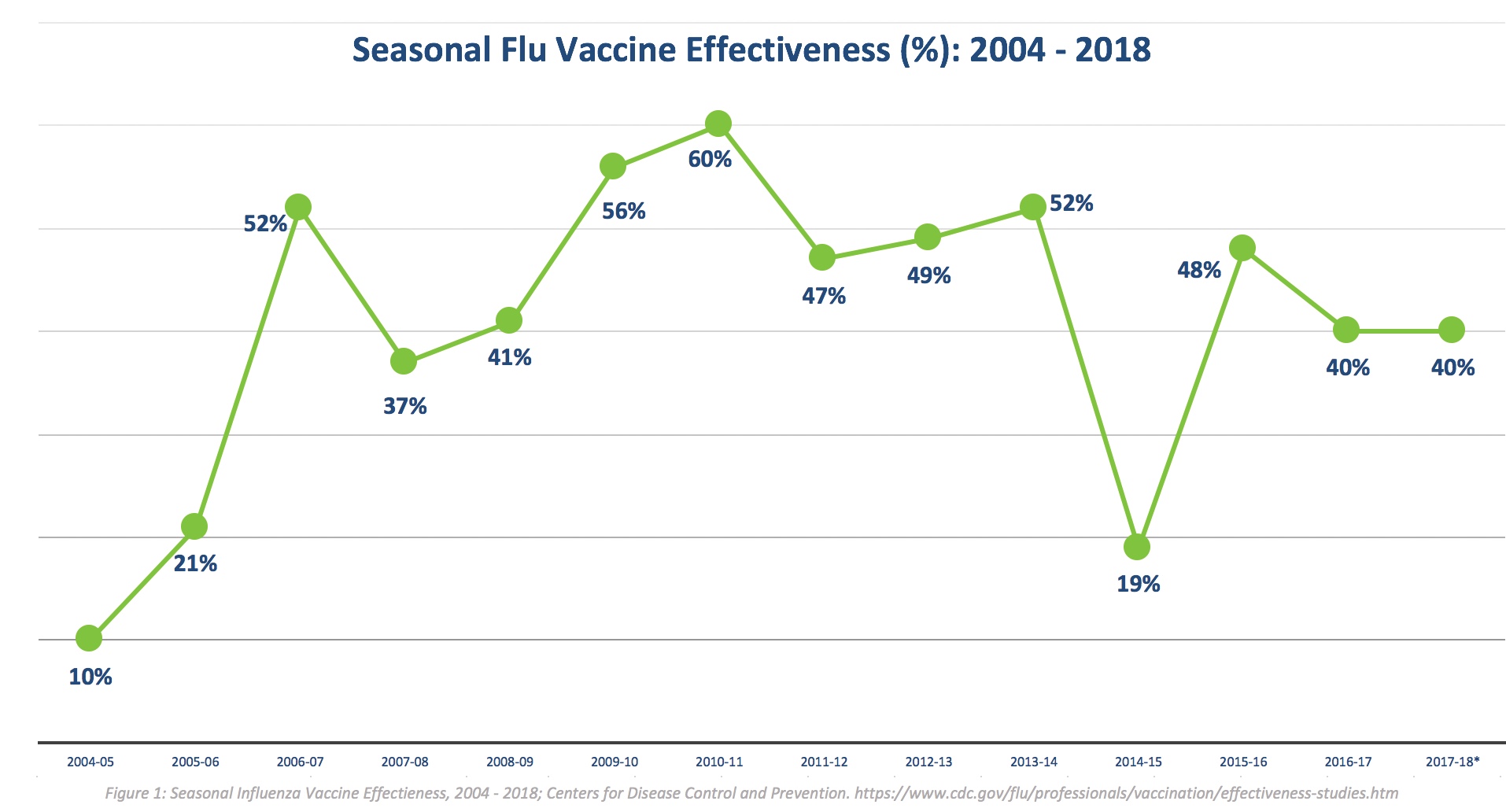
In the FluGen study, volunteers were given a single intranasal dose of either placebo or the M2SR vaccine. Next, they were deliberately challenged with the A/Belgium/4217/2015, H3N2 virus, which had been controlled with only 19% overall effectiveness in that year due to a high degree of mismatch with that year’s flu vaccine. In fact, the mismatch between the A/Belgium/4217/2015, H3N2 virus and the M2SR vaccine (which was based on the A/Brisbane/10/2007, H3N2 strain) was even higher, and yet viral load was reduced by 34% in participants in the M2SR group compared to placebo, with 51% reduction in symptom scores. Participants who produced antibody responses to both the M2SR vaccine and the A/Belgium/4217/2015, H3N2 virus had a 62% reduction in viral load and 56% reduction in symptom scores.
These results are extremely encouraging, considering that standard flu vaccination is not protective from strain to strain or year to year, and that immunoprotective reactions decline with the age of the patient receiving the vaccine.
New Methods of Vaccine Production Will Require Careful Regulatory Compliance to Ensure Safety
Another important aspect of the work, which will be watched with interest, is the method of vaccine production. Instead of expressing the antigen proteins in chicken eggs, FluGen grows its single-replication viruses in cell culture. Growing dependence on mammalian cell culture for production of therapeutic proteins, vaccines and other molecules will increase regulatory pressure for biomanufacturers to source pure, reliable, and pathogen-free cell culture components that minimize the chance of cross-contamination with other viruses or prion pathogens.
This story is only just beginning. We will continue to share more news and analysis here as it unfolds.