- Home
- High-Quality Recombinant Human Serum Albumin (rHSA), Exbumin™, for Improved Cell Wash Buffer Preparation
High-Quality Recombinant Human Serum Albumin (rHSA), Exbumin™, for Improved Cell Wash Buffer Preparation
Published on 18 January 2023
Lila Ross, Product Application, Technical Lead at InVitria
Introduction
Cell therapy offers a novel approach to medicine, leveraging the body’s own cellular machinery to treat a variety of indications in regenerative medicine, immune disease, and cancer.1 Since the mid 1990s, scientists have been exploring ways to create robust and reliable manufacturing systems leveraging high-quality, well-defined, GMP (Good Manufacturing Practice)-grade raw materials.2,3 Traditionally sourced components such as serum and serum-derived proteins pose a unique challenge to this initiative. Specifically, human and bovine serum and serum albumin (HSA & BSA) leveraged in downstream processing buffers can create challenges in the manufacturing, formulation, and regulatory approval process. Strategies have been developed to replace these undefined components with recombinant alternatives to improve performance and supply chain continuity, and reduce the risk of using animal or human (i.e. XENO-free) derived components in clinical applications.
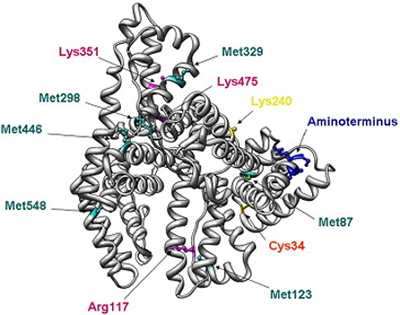
A typical cell therapy manufacturing process involves cell isolation and activation, transduction, and expansion of genetically modified cells, to downstream processing where cells are washed, concentrated, and formulated in a buffer and/or cryopreservation media for patient administration.4 Because cell therapies cannot be sterile-filtered as with traditional pharmaceuticals, alternative methods for downstream processing are employed to recover the valuable cell product while maintaining cell quality, therapeutic potency, and stability.3
Two main methods for cell washing and concentration are centrifugation and tangential flow filtration (TFF).3 When choosing the right approach, manufacturers consider multiple factors including culture volume, processing time, shear sensitivity, and desired cell count.3 Key characteristics of the final cell product are also monitored such as viability. During these processing steps, cells are subjected to a variety of environmental insults including shear stress, cell aggregation, and non-specific binding to vessel walls. Manufacturers have a limited amount of time once the cells are removed from their nutrient-dense environments, either from the body or expansion media, and placed in processing buffer solutions.
Protein Protection
In order to maximize cell recovery and maintain cell function during downstream processing, a protein source is often leveraged in the buffer preparations. Common processing buffer formulations contain a mixture of saline solution (i.e., PBD, DPBS, PlasmaLyte) and whole serum or serum-derived albumin. Drug product formulations may also contain cryopreservation solvents like DMSO,5 although recent strategies have been developed to remove DMSO from the manufacturing process to enhance cell potency and reduce concerns around using a strong organic solvent that may increase extractables and leachables in single-use tubing and plastics used during clinical manufacturing (see OptiFRZ). The final cryopreserved cell product can be thawed and administered to the patient or washed and resuspended in another saline and HSA solution for administration.5 Such is the case with many commercialized cell therapies like Novartis’s Kymriah® and Kite’s Tecartus® and Yescarta®.6,7,8
It is widely accepted that albumin provides protection from shear stress, prevents cell aggregation, and mitigates non-specific adsorption to tubing, vessels, and equipment surfaces. One example is Kikuchi et al demonstrating BSA in no to low serum-containing culture media prevented cell adhesion to both treated and untreated polystyrene surfaces.10 The same property is applied to processing buffers to prevent cell adhesion and aid recovery during purification and concentration. With this in mind, the incorporation of albumin into downstream processing buffers can serve a beneficial role in improving high-quality cell recovery and stability in formulation.
The Unnecessary Risks
While albumin is a key component in these processing buffers, serum-derived albumin preparations can pose a threat to manufacturing consistency, risk mitigation, and supply chain continuity. Lot-to-variabilities in serum protein chemistry can also introduce unnecessary variability and potential inconsistencies in the manufacturing process. A comparison of five commercial serum-derived HSA preparations showed significant variability in post-translational modifications, including indicators of protein function.11 Concerns over pathogenic contaminants (e.g., viruses, prions, BSE/TSE) also pose a safety risk for patients and a risk to manufacturing facilities that supply these life-saving cell therapies.12
In addition, serum proteins are not limitless, and the biologics industry is experiencing a critical and urgent tightening of supply chain capabilities to meet the ongoing demand. To date, there have been 9 cell therapies approved in the United States.13 As of 2021, the global cell therapy pipeline includes roughly 1,350 active clinical cell therapy trials, an increase of 78% from 2019.12 The FDA has stated they expect an average of 10-20 annual cell therapy approvals by 2025.14 Taking one of the commercially approved cell therapies, Kymriah® as an example, the inclusion levels of albumin in therapy ranges from 0.5g-2.5g per dose.6 The number of injections of Kymriah® is now up to over 5,300 successful administrations.15 Taking the average dose of ≈1.25g HSA and applying the number of active clinical trials with potential target populations, roughly 9100 kgs of albumin will be needed. This does not consider cell therapies for diseases such as Parkinson’s and Dementia that have patient populations in the millions.16,17 The current human/bovine serum supply chain capacity is not set up to meet the needs of this rapidly growing field and more scalable alternatives are needed to continue down the path of incredible innovation.
A Better Alternative
Recombinant human serum albumin can be a safe, consistent, and well-defined alternative to its serum counterpart, facilitating more reliable and scalable cell therapy manufacturing processes. InVitria leverages a plant-based expression system to produce high-quality recombinant albumin at large scale that eliminates the unnecessary risks associated with serum-derived albumin. Leveraging the earth’s natural resources, i.e., sunlight, water, and soil, the highly scalable and sustainable system can respond rapidly to increasing demand while eliminating the risk of adventitious agents. The recombinant technology also removes lot-to-lot inconsistencies, facilitating a more reliable manufacturing process. InVitria’s excipient-grade recombinant human serum albumin, Exbumin™, is the first and only cGMP, excipient recombinant albumin approved for use in biologics by the FDA and EMA. It is currently in the formulation of biologics used worldwide such as Merck’s ERVEBO vaccine.19 Exbumin can facilitate the replacement of serum-derived albumin in downstream processing formulations and advance innovations in chemically defined, animal-origin-free cell therapy manufacturing.
Summary
Human and bovine serum albumin have been integral to cell therapy manufacturing for years, given its unique chemical properties and benefits to cell populations during manufacturing. Both downstream cell processing buffers utilize albumin for a variety of functions, however, animal and human-derived components pose a serious risk to the safety, quality, and consistency of final drug products. The need for an alternative source is imperative given the proliferation of cell therapy programs. InVitria’s plant-based recombinant protein production system allows for high mass production, incredible scale-up capabilities, eliminated risk of human pathogens, and high-performance consistency in cell therapy manufacturing process. Exbumin ™ presents a regulatory friendly alternative that can support growing cell therapy programs globally.
Learn more at https://invitria.com/products/exbumin-rhsa-excipient/ or fill out a Contact Us Form to speak with one of our Product Applications Team members.
| 2718 Industrial Drive
Junction City, KS 66441 |
Phone: 1.800.916.8311
Email: Info@InVitria.com |
The following content is gated. Please, subscribe to open access to it.
Footnotes
- El-Kadiry, A. E.-H., Rafei, M., & Shammaa, R. (2021, November 1). Cell therapy: Types, regulation, and clinical benefits. Frontiers. Retrieved January 5, 2023, from https://doi.org/10.3389/fmed.2021.756029
- Immel, B. (2002, November). A Brief History of the GMPs. Immel Resources LLC. Retrieved January 5, 2023, from http://immelresources.com/HistoryofGMPs.pdf
- Campbell, A., Brieva, T., Raviv, L., Rowley, J., Niss, K., Brandwein, H., Oh, S., & Karnieli, O. (2015). Concise review: Process development considerations for cell therapy. Stem Cells Translational Medicine, 4(10), 1155–1163. https://doi.org/10.5966/sctm.2014-0294
- Kaiser, A. D., Assenmacher, M., Schröder, B., Meyer, M., Orentas, R., Bethke, U., & Dropulic, B. (2015). Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Therapy, 22, 72–78. https://doi.org/10.1038/cgt.2014.78
- LI, R. U. I., JOHNSON, R. A. C. H. E. L., YU, G. U. A. N. G. L. I. N., MCKENNA, D. A. V. I. D. H., & HUBEL, A. L. L. I. S. O. N. (2019). Preservation of cell-based immunotherapies for clinical trials. Cytotherapy, 21(9), 943–957. https://doi.org/10.1016/j.jcyt.2019.07.004
- KYMRIAH [package insert]. Food and Drug Administration: Novartis Pharmaceuticals Corporation; 2022.
- TECARTUS [package insert]. Food and Drug Administration: Kite Pharma, Inc.; 2021
- YESCARTA [package insert]. Food and Drug Administration: Kite Pharma, Inc.; 2022
- Kwok, Y. K., Tang, M. H., Law, H. K., Ngai, C. S., Lau, Y. L., & Lau, E. T. (2007). Maternal plasma or human serum albumin in wash buffer enhances enrichment and ex vivo expansion of human umbilical cord blood CD34+ cells. British Journal of Haematology, 137(5), 468–474. https://doi.org/10.1111/j.1365-2141.2007.06606.x
- Kikuchi, T., Matsuura, K., & Shimizu, T. (2021). Non-coating method for non-adherent cell culture using high molecular weight dextran sulfate and bovine serum albumin. Journal of Bioscience and Bioengineering, 132(5), 537–542. https://doi.org/10.1016/j.jbiosc.2021.08.006
- Miyamura, S., Imafuku, T., Anraku, M., Taguchi, K., Yamasaki, K., Tominaga, Y., Maeda, H., Ishima, Y., Watanabe, H., Otagiri, M., & Maruyama, T. (2016). Comparison of posttranslational modification and the functional impairment of human serum albumin in commercial preparations. Journal of Pharmaceutical Sciences, 105(3), 1043–1049. https://doi.org/10.1016/j.xphs.2015.12.015
- Gupta, V., Sengupta, M., Prakash, J., & Tripathy, B. C. (2016). Production of recombinant pharmaceutical proteins. Basic and Applied Aspects of Biotechnology, 77–101. https://doi.org/10.1007/978-981-10-0875-7_4
- Center for Biologics Evaluation and Research. (2022, March). Considerations for the development of (CAR) T cell products. U.S. Food and Drug Administration. Retrieved January 5, 2023, from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-development-chimeric-antigen-receptor-car-t-cell-products
- Gottlieb, S. (2019, January 15). Statement from FDA commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.d., director of the Center for Biologics Evaluation and research on new policies to advance development of safe and effective cell and Gene Therapies. U.S. Food and Drug Administration. Retrieved January 5, 2023, from https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics
- Liu, A. (2021, August 24). Novartis’ kymriah flops in earlier lymphoma use, where Gilead, Bristol Myers Car-T Rivals succeeded. Fierce Pharma. Retrieved January 5, 2023, from https://www.fiercepharma.com/pharma/novartis-loses-car-t-race-for-earlier-lymphoma-as-kymriah-fails-where-gilead-bristol-myers