- Home
- Maintaining Viable Cell Count in Primary T Cells During Cell Washing Using Optibumin®
Maintaining Viable Cell Count in Primary T Cells During Cell Washing Using Optibumin®
Published on 4 October 2024
Application Note
Mark Stathos, PhD, Product Applications Scientist, InVitria, Inc.
Key Points
- Optibumin®, InVitria’s high-purity recombinant human albumin, performs comparably to serum-derived albumin in cell wash experiments, retaining nearly all cells and maintaining excellent viability after washing.
- Optibumin offers the additional benefits of animal-free origin and consistent quality.
Introduction
Downstream cell washing is a critical step in cell therapy manufacturing processes (Figure 1). During this step, it is vital to remove media components that are not suitable for final formulation or cryopreservation while also maintaining cell count and viability during prolonged hold times (Li, 2021; Ibenena, 2022). Albumin is a key component of buffers used to accomplish this due to its ability to protect cells from oxidation (Lu, 2016) and mechanical stress (Shahin, 2020), its capacity to bind and remove unwanted components (Wang, 2022; Hoogenboezem 2018) and its cryoprotective properties (Lu, 2016). Due to these benefits, albumin is often included in the final formulation of cell therapy drug products such as Yescarta® (Gilead Sciences®, 2024). However, the albumin used in cell therapy is often derived from human serum which presents issues with product safety and uniformity (Merten, 1999). To address these issues, InVitria® has developed Optibumin, a recombinant albumin produced in a non-mammalian expression host and tested its performance in a cell wash application alongside a clinical human serum albumin (HSA) product that is often used in cell therapy manufacturing processes.
Results and Discussion
Cell Retention
After activation, aliquots of 1% and 5% Optibumin or HSA were prepared in PBS or Plasma-Lyte backgrounds for washing along with PBS only, Plasma-Lyte only, and cell culture media as controls. Primary human T cells were then counted, aliquoted at a uniform density, washed by centrifugation in the various conditions described above, and counted again. Centrifugation is an industrially relevant unit operation for performing cell washes (Li, 2021; Ibenena, 2022). The portion of cells retained was calculated for each condition (Figure 2). The data shown represents the average of three donors. In a Plasma-Lyte background, Optibumin showed improved cell retention compared to HSA at both 1% and 5% concentrations. Retention was comparable across albumin types in a PBS background with 1% albumin conditions outperforming 5% albumin. Cell retention was greater than 80% for all conditions. This is encouraging because high cell retention during wash is vital for ensuring proper dosing during final formulation a maximizing process efficiency.
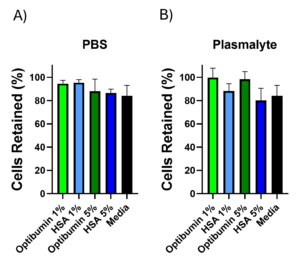
The percentage of the initial cell count retained after three washes in A) PBS or B) Plasma-Lyte supplemented with the indicated concentrations of Optibumin or HSA. No significant differences were found using one-way ANOVA, α = 0.05.
Cell Viability
To assess the effect of wash buffer composition on T cell viability, T cells were seeded into a 96-well plate at a uniform density with a viability dye added and viability measured well-by-well using high content screening. Mean initial viability was 92% with a standard deviation of 1%. The plated cells were then washed by centrifugation in the same buffers described in the cell retention experiment and their viability measured well-by-well again post-wash using the same viability dye. Changes in viability (Figure 3) were calculated across all three donors. These changes were less than 5% for all conditions and albumin wash buffers performed comparably to the media wash control. This suggests that albumin buffers are able to prevent cell death during processing which is important for cell therapy applications.
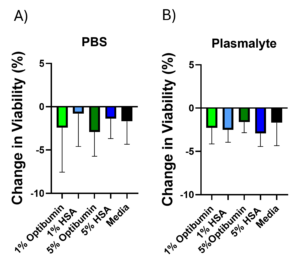
Decrease in cell viability after washing in buffers containing A) PBS or B) Plasma-Lyte, supplemented with the indicated concentrations of Optibumin or HSA. No significant differences were found by one-way ANOVA, α = 0.05.
Optibumin shows almost identical cell retention throughout the washing process compared to serum HSA in PBS and slightly better in Plasma-Lyte backgrounds. Cells treated with Optibumin also showed similar viability after the wash and hold process, which was comparable to the performance of HSA. The comparability between Optibumin and HSA is not surprising and is likely due to albumin’s ability to protect cells from shear stress during washes and provide a nutrient source during the prolonged hold times. Optibumin’s performance in these experiments is notable because washing cells by centrifugation and pipetting is more stressful than washing with a specialized device therefore this dataset demonstrates that Optibumin performs well even in especially stressful processes applicable to cell washing.
Conclusion
The cell wash experiments described herein demonstrate that Optibumin performs comparably to HSA in cell therapy wash applications in all key metrics. It prevents cell loss during the washing process and maintains cell viability and health to a similar extent as HSA. In addition to this, it has the added safety benefit of being animal origin free and the consistency of a manufactured product free from donor variability. These advantages make Optibumin a superior alternative for clinical cell wash applications.
Materials and Methods
Primary Human T Cell Isolation, Activation, and Culture
Primary T cells were isolated from leukopaks derived from 3 healthy non-smoker donors between 18 and 65 years of age with BMI <30 using a negative selection kit, following the manufacturer’s instructions. The isolated cells were cryopreserved in liquid nitrogen in a formulation containing 10% DMSO. The cells were later thawed and adjusted to a density of 1.0 × 10⁶ cells/mL in serum-free T cell media supplemented with 200 IU/mL of IL-2. A CD3 and CD28 antibody cocktail was added to the cells for three days to activate them.
Cell Retention Assessment
After three days of activation, 10 mL aliquots of the wash buffer conditions described above were prepared. The primary human T cells were counted using an automated cell counter and aliquots of 2.0 × 10⁵ live cells were transferred to microfuge tubes. The cells were pelleted by centrifugation at 500 × g for 5 minutes. The supernatants were aspirated, and the cell pellets were resuspended in 1 mL of each wash buffer. This washing procedure was repeated two more times, for a total of three washes. During the final wash, the cells were held in the wash buffer for 2 hours to mimic a typical hold time in a cell therapy manufacturing process (Pattasseril, 2013). The cells were then resuspended in cell culture media and counted again using the same automated cell counter.
Cell Viability Assessment
T cells were counted using an automated cell counter and seeded into sterile 96-well F-bottom plates at 2.0 × 10⁴ live cells/well. The cells were initially imaged using a high-content screening device to assess viability in each well, with a mean initial viability of 92%. The cells were then transferred to 96-well V-bottom plates and centrifuged at 500 × g for 5 minutes at room temperature. The supernatants were carefully removed using a 12-channel pipette, taking care to minimize disruption of the pellets, and the cells were washed three times with 200 µL of the same wash buffers used in the cell retention experiment. During the final wash, the cells were held in the buffer at room temperature for 2 hours. The cells were then plated at 2.0 × 10⁴ cells/well in 96-well flat-bottom plates in serum-free media containing an NIR cytotoxicity stain, following the manufacturer’s instructions. Cell viability was assessed before and after washing using high-content screening. Cells were gated into high and low stain subpopulations in a cell-by-cell manner, based on the mean fluorescence intensity of each cell identified in the phase channel by the analysis software.
The following content is gated. Please, subscribe to open access to it.
Footnotes
References
- Li, A., Kusuma, G. D., Driscoll, D., Smith, N., Wall, D. M., Levine, B. L., James, D., & Lim, R. (2021). Advances in automated cell washing and concentration. Cytotherapy, 23(9), 774–786. https://doi.org/10.1016/j.jcyt.2021.04.003
- Ibenana, L., Anderson, R., Gee, A., Gilbert, M., Cox, C., Hare, J. M., Brooks, A., Kelley, L., Khan, A., Lapteva, N., Orozco, A., Styers, D., Sumstad, D., Ugochi, I., & McKenna, D. H. (2022). Assessment of the LOVO device for final harvest of novel cell therapies: A Production Assistance for Cellular Therapies multi-center study. Cytotherapy, 24(7), 691–698. https://doi.org/10.1016/j.jcyt.2022.01.010
- Lu, T. L., Pugach, O., Somerville, R., Rosenberg, S. A., Kochenderfer, J. N., Better, M., & Feldman, S. A. (2016). A Rapid Cell Expansion Process for Production of Engineered Autologous CAR-T Cell Therapies. Human gene therapy methods, 27(6), 209–218. https://doi.org/10.1089/hgtb.2016.120
- Gilead Sciences. (2024) Medication Guide Yescarta Gilead Sciences. Retrieved from https://www.gilead.com/-/media/files/pdfs/medicines/oncology/yescarta/yescarta_medguide.pdf
- Shahin, H., Elmasry, M., Steinvall, I., Markland, K., Blomberg, P., Sjöberg, F., & El-Serafi, A. T. (2020). Human serum albumin as a clinically accepted cell carrier solution for skin regenerative application. Scientific Reports, 10(1), 14486. https://doi.org/10.1038/s41598-020-71553-2
- Wang, H., Tsao, ST., Gu, M. et al. (2022). A simple and effective method to purify and activate T cells for successful generation of chimeric antigen receptor T (CAR-T) cells from patients with high monocyte count. Journal of Translational Medicine, 20, 608. https://doi.org/10.1186/s12967-022-03833-6
- Hoogenboezem, E. N., & Duvall, C. L. (2018). Harnessing albumin as a carrier for cancer therapies. Advanced Drug Delivery Reviews, 130, 73–89. https://doi.org/10.1016/j.addr.2018.07.011
- Merten O. W. (1999). Safety issues of animal products used in serum-free media. Developments in Biological Standardization, 99, 167–180.
- Pattasseril, J., Varadaraju, H., Lock, L., Rowley, J. (2013) Downstream Technology Landscape for Large-Scale Therapeutic Cell Processing. BioProcess International, 11(3)s, 38-47
Copyright © 2024 InVitria, Inc. All rights reserved.
Reproduction or distribution of any InVitria materials, in whole or in part, is prohibited without prior written consent. All logos, names, designs, and marks displayed, including InVitria®, the InVitria® brand design and Optibumin®, are trademarks or service marks owned or licensed by InVitria, Inc., Kansas, USA, unless otherwise noted. For details on InVitria’s registered intellectual property, patents, and additional terms and conditions, please visit www.InVitria.com/terms.