- Home
- Process Optimization for Lentivirus Production in the iCELLis® Nano Using a Chemically Defined, Animal Component Free HEK293 Media
Process Optimization for Lentivirus Production in the iCELLis® Nano Using a Chemically Defined, Animal Component Free HEK293 Media
Published on 12 April 2022
Written by Sofia Pezoa, PhD, Director of Cell Culture, InVitria
Sofia Pezoa is Director of Cell Culture team at InVitria where she works on product development for the manufacturing of virus-based vectors for gene therapies and vaccines. Prior to joining InVitria, Sofia earned her PhD from the University of Colorado Anschutz in the Cell Biology, Stem Cell, and Developmental Biology graduate program.
Abstract
Use of FBS in large scale clinical manufacturing applications represents a significant risk due to potential adventitious agent contamination, unreliable supply chain from limited global supply, and considerable variability in vector yield due to lot-to-lot composition variations. To circumvent the many challenges and drawbacks associated with using serum in viral vector production media for adherent cells, we utilized recombinant human proteins to formulate a chemically defined, serum-free and animal component free media that is optimized specifically for production of viral vectors in 293 and 293T cells in the iCELLis fixed bed bioreactor, called OptiPEAK HEK293t®. We therefore performed optimization studies in OptiPEAK HEK293t to determine the optimal parameters for LV production in the iCELLis Nano bioreactor. The parameters tested included cell density at the time of transfection, linear speed post transfection, pH as well as Dissolved Oxygen (DO) post transfection. Our results demonstrate that high cell densities at the time of transfection were negatively correlated with virus titer. We also observed that a high linear feed rate can be used post transfection without compromising virus titer. Taken together, we have demonstrated that the iCELLis Nano Bioreactor can be used with ACF conditions to achieve high lentivirus titer for clinical manufacturing.
Background
Clinical therapies utilizing viral vectors for the treatment of rare diseases or the generation of modified T cells for cancer treatment is a promising therapeutic with increasing clinical success. Manufacturing of viral vectors with adherent HEK293 or HEK293T cells routinely includes basal media supplemented with 10% fetal bovine serum (FBS) for both growth and production of viral vectors. While FBS inclusion supports growth and vector production, there are still considerable safety concerns around the use of FBS, as well as concerns about the use of FBS in large scale manufacturing. Furthermore, requirements to show that FBS has been removed in the final vector product can hinder advancements to clinical trials. Despite the safety concerns surrounding FBS, there is limited data surrounding the manufacturing capabilities with adherent HEK cells using medium that is completely free from serum or animal derived components. Although FBS is undefined chemically and contains unknown levels of growth factors or proteins, several components within FBS have been identified to be critical for cell growth and attachment. Therefore, we utilized recombinant human proteins to formulate a chemically defined, animal component free (ACF) medium that supports adherent HEK cell growth and viral vector production, called OptiPEAK HEK293t®.
Our previous results indicated that OptiPEAK HEK293t yields high titer lentivirus in flatware. To demonstrate that OptiPEAK HEK293t can be used for clinical manufacturing, we scaled our flatware process to the iCELLis Nano bioreactor. The iCELLis bioreactor technology supports HEK adherent vector production and is scalable to large scale formats with the iCELLis 500. Although the technology is scalable for clinical manufacturing, set point parameters for viral vector production have not been intensively investigated. In addition, reported yields for lentivirus in the iCELLis bioreactors were significantly lower than the titers achieved in flatware, suggesting that either the bioreactor does not support lentivirus production or that set point parameters were sub-optimal for lentivirus production. We thus hypothesized that set point parameters can be optimized in the iCELLis bioreactor in order to achieve high titer lentivirus. We utilized OptiPEAK HEK293t in the iCELLis bioreactor and optimized transfection density, linear speed, pH, and dissolved oxygen (DO) to achieve high lentivirus titer. Our results demonstrate that a serum free, ACF process can be incorporated in manufacturing methods for clinical production of viral vectors.
Methods
Cell Lines and Culture Methods
HEK293T and HT-1080 (ATCC Catalog CRL-3216 and CCL-121) were maintained in high glucose DMEM with GlutaMAX (Gibco, Grand Island, NY) supplemented with 10% US-sourced FBS (Gibco, Grand Island, NY), for HT1080 or in OptiPEAK HEK293t (InVitria, Junction City, KS) for HEK293T. OptiPEAK HEK293t cultured cells were maintained in a 10% CO2 and 95% humidity environment on CellBIND® flasks (Catalog #3290, Corning, Corning, NY). Cells were maintained by passaging twice a week and seeding at a density of 10,000 cells per cm2. Cell density and viability were determined by trypan blue exclusion using a hemocytometer.
iCELLis Fixed Bed Bioreactor Runs for Lentivirus Production
iCELLis bioreactors (0.53m2, Pall Corporation, Westborough MA) fitted with biomass probes (Aber, Arlington VA) and DO and pH probes were assembled and calibrated per the manufacturer’s instructions. The biomass probe was set to read capacitance in pF/cm every 40 ms and the resulting capacitance was correlated to cell density using the equation of Cell density (cells/cm2) = 928.47 * capacitance. This equation was determined by linear regression of the correlation between nuclei counts and capacitance from non- virus producing bioreactor runs (Laskowski et al., 2019). Bioreactors were sterilized by autoclave and then batched with 650 mL of OptiPEAK HEK293t, unless otherwise stated, 24 hours prior to cell inoculation to ensure complete wetting of the cell attachment surface and normalization of biomass probe conductivity. Immediately prior to inoculation, biomass probes were set to zero and cell inoculation was considered to be Time = 0 (T0). Bioreactor parameters were monitored online via BioXpert SCADA software and spent media was collected from the bioreactors once daily to measure media metabolites, and offline pH. Bioreactor setpoints prior to transfection were maintained at 37 °C, pH 7.25, linear speed rate of 2 cm/s, and 95% DO or 55% DO for serum conditions, unless otherwise stated. Bioreactors were transfected with LV vector plasmids by transfecting with OptiPEAK HEK293t supplemented with 1 mg/mL recombinant transferrin (Optiferrin, InVitria, Junction City, Kansas). 10% of the working volume was used for complexation, then 650mL of the transfection media including transfection complexes were added to the bioreactors by a complete media exchange. Bioreactors were transfected for 2 hours while maintaining bioreactor set points. Post transfection, bioreactors underwent a complete media change into full growth media and bioreactors were run for an additional 72 hours. LV vectors were harvested by collecting the complete supernatant from the bioreactors, clarified by centrifugation, and immediately used for functional titering.
Functional Titering
HT-1080 cells were plated in 12-well plates at 30,000 cells/well and allowed to adhere for 2 hours at normal growth conditions. For GFP transduction, Lenti-pSIH1-H1-siLuc-copGFP were titrated in infection medium consisting of DMEM+10% FBS supplemented. Transductions using the Lenti-pSIH1-H1-siLuc-copGFP were done in the presence of 10 µg/mL polybrene (Millipore Sigma, St. Louis, MO). Lentivirus vectors were titered as previously described (Sena-Esteves & Gao, 2018). At the conclusion of the subculture time, cells were harvested with 1x TrypLE + 1 mM EDTA and enzyme was quenched with 10% FBS in DPBS (ThermoFisher Cat. #1404141). Cells were pelleted and resuspended in DPBS supplemented with 10% FBS, and 1x F-68 (ThermoFisher, Cat. #24040032). GFP expression was determined by flow cytometry.
Results and Conclusions
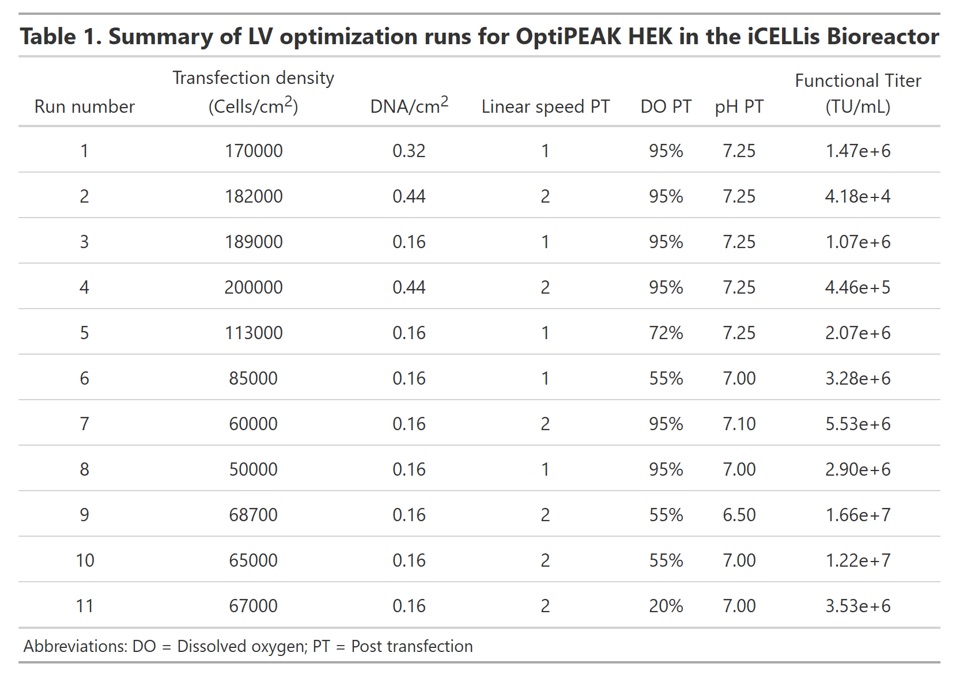
A total of 11 iCELLis Nano bioreactors were run to determine the optimal conditions for high titer lentivirus production in ACF medium (Summarized in Table 1). We initially hypothesized that a high transfection density would yield more virus producing cells post transfection. Therefore, the iCELLis bioreactors were inoculated at a cell density of 10,000 cells/cm2 and transfected 3 days post inoculation. On the day of transfection, the cell density was greater than 170,000 cells/cm2. The amount of plasmid DNA delivered was also increased to account for the increased biomass. Functional titer of lentivirus produced yielded very low titers, with functional titers of 2e+5 TU/mL or less (Table 1). We thus repeated this experiment with a lower amount of DNA delivered per cm2 compared to a DNA concentration of almost three-fold higher to determine if high amounts of DNA or PEI are cytotoxic. DNA was delivered at either 0.16 µg/cm2 or 0.44 µg/cm2 while maintaining the cell density at approximately 170,000 cells/cm2. The lowest DNA amount yielded a modest improvement over the first experiment; however, the functional titer was still low at approximately 1.07e+6 TU/mL (Table 1). A drop in viability was not observed with the higher DNA concentration as determined by the online biomass probe; we thus concluded that the high cell density was inhibiting transfection efficiency through either contact inhibition or cell clumping. We performed further optimization studies with target transfection cell densities three-fold lower than the first two experiments and addressed the post-transfection parameters of linear speed rate, DO, and pH while maintaining the target cell density at the time of transfection between 50,000 and 100,000 cells/cm2 (Runs 6-11). Total production time post transfection was 72 hours and virus production was run without perfusion.
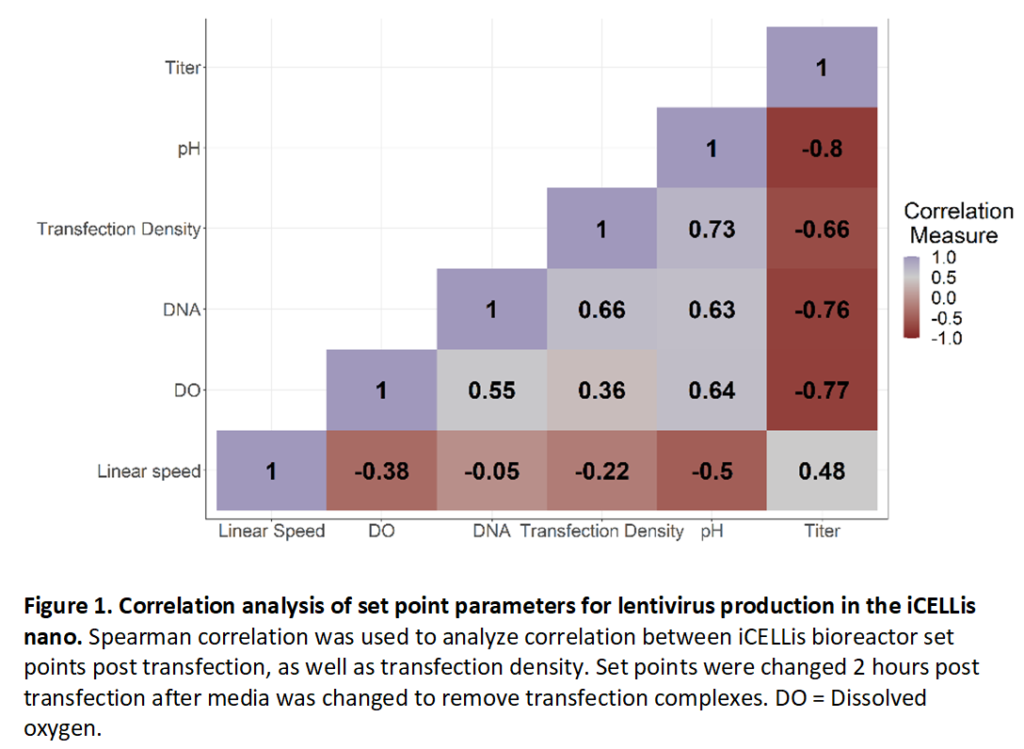 Functional titer from bulk harvest 72 hours post transfection was then analyzed by Spearman correlation to determine if there was any correlation between set point parameters post transfection and lentivirus titer (Figure 1). Transfection density (cells/cm2), DNA concentration for transfection, and DO were all strongly negatively correlated with viral titer, suggesting that higher density of cells/cm2 at transfection or high amounts of DNA delivered for transfection negatively impact viral titer possibly through low transfection efficiency (Figure 1). DO was also negatively correlated, suggesting that cells do not consume large amounts of oxygen during viral production. A pH greater than 7.1 was strongly negatively correlated with titer, and high titers were achieved at pH values less than 7.0, consistent with previous observations that low pH helps to stabilize the lentivirus membrane (Holic et al., 2014). Of the different post-transfection parameters tested, a higher linear speed rate of 2 cm/s was positively correlated with virus titer. This data suggests that either, VSV-G pseudotyped lentivirus is not sensitive to shear stress in the iCELLis Nano, or that the higher feed rate through the fixed bed improved virus production by increasing nutrient distribution when compared to a linear speed of 1 or lower.
Functional titer from bulk harvest 72 hours post transfection was then analyzed by Spearman correlation to determine if there was any correlation between set point parameters post transfection and lentivirus titer (Figure 1). Transfection density (cells/cm2), DNA concentration for transfection, and DO were all strongly negatively correlated with viral titer, suggesting that higher density of cells/cm2 at transfection or high amounts of DNA delivered for transfection negatively impact viral titer possibly through low transfection efficiency (Figure 1). DO was also negatively correlated, suggesting that cells do not consume large amounts of oxygen during viral production. A pH greater than 7.1 was strongly negatively correlated with titer, and high titers were achieved at pH values less than 7.0, consistent with previous observations that low pH helps to stabilize the lentivirus membrane (Holic et al., 2014). Of the different post-transfection parameters tested, a higher linear speed rate of 2 cm/s was positively correlated with virus titer. This data suggests that either, VSV-G pseudotyped lentivirus is not sensitive to shear stress in the iCELLis Nano, or that the higher feed rate through the fixed bed improved virus production by increasing nutrient distribution when compared to a linear speed of 1 or lower.
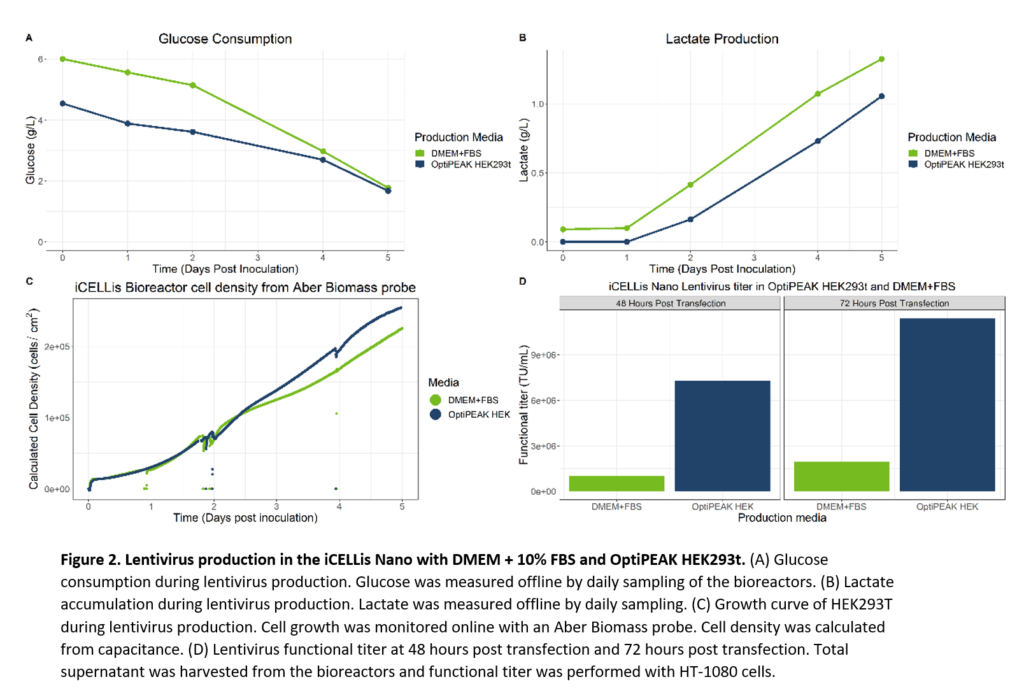 Final evaluations of lentivirus production included a comparison of virus production in the iCELLis Nano bioreactor with iCELLis bioreactors batched with either OptiPEAK HEK293t or DMEM containing 10% FBS. Two 0.53m2 iCELLis Nano bioreactors were inoculated with HEK293T serially passaged in OptiPEAK HEK293t or DMEM + 10% FBS.
Final evaluations of lentivirus production included a comparison of virus production in the iCELLis Nano bioreactor with iCELLis bioreactors batched with either OptiPEAK HEK293t or DMEM containing 10% FBS. Two 0.53m2 iCELLis Nano bioreactors were inoculated with HEK293T serially passaged in OptiPEAK HEK293t or DMEM + 10% FBS.
Both iCELLis bioreactors were inoculated at 10,000 cells/cm2 and transfected at day 2 post inoculation. Initial experiments done in flatware indicated that HEK293T serially passaged in DMEM + 10% FBS and transfected with the same DNA and transfection densities optimized from the iCELLis Nano optimization yielded high titers within the standard deviation of lentivirus produced in OptiPEAK HEK293t. We thus applied the same transfection parameters and post-transfection set-points optimized for lentivirus to the iCELLis Nano for lentivirus production with both OptiPEAK HEK293t and DMEM + 10% FBS media. Total supernatant from bioreactors was harvested at 48 hours and 72 hours post transfection, clarified, and then immediately used for functional titer. Glucose consumption for both media over the course of lentivirus production had similar trends, albeit DMEM started at a higher glucose level initially as the media is formulated as high glucose (Figure 2, A). Lactate trends were also similar, with again DMEM + 10% FBS starting at a higher lactate level due to the presence of lactate in FBS (Figure 2, B). While lactate at high concentrations can be toxic to cells, initial lactate exposure at lower levels does not have any known effect. Growth trends between the two media in the iCELLis bioreactors were also very similar (Figure 2, C). At the time of transfection, the cell density (cells/cm2) for both bioreactors was roughly 80,000 cells/cm2, indicating that for both media the cells would receive the same plasmid DNA per cell. Despite the same growth kinetics and transfection densities, the functional titer from the iCELLis bioreactor with DMEM + 10% FBS yielded moderate titers of 2.6e+6 TU/mL at the end of production, 72 hours post transfection (Figure 2, D). Final functional titer at the end of production was 1.07e+7 TU/mL in OptiPEAK HEK293t, approximately three-fold higher than the titers produced with DMEM + 10% FBS. Taken together, the high titer achieved with OptiPEAK HEK293t also suggests that lentivirus is not shear sensitive in the iCELLis nano, and the increased linear speed rate improved production.
Our results here demonstrate that lentivirus can be produced with HEK293T in media that is formulated without serum or animal derived components. To demonstrate that lentivirus can be produced using formats representative of large-scale manufacturing, we performed lentivirus production in the iCELLis Nano bioreactors as the iCELLis bioreactor technology is scalable to large scale formats with the iCELLis 500. Our results indicate that set-point parameters can be optimized to achieve high titer lentivirus, and that transfection density and pH post-transfection were the two most critical points to achieve high titer with ACF media, OptiPEAK HEK293t. It is unclear why DMEM + 10% FBS did not yield high titer in the iCELLis Nano. While our process was optimized for OptiPEAK HEK293t, in flatware conditions we have achieved comparable results with DMEM + 10% FBS and OptiPEAK HEK293t. It remains to be tested if the increased protein content or undefined cholesterol levels can negatively affect viral titer in the iCELLis bioreactor, and furthermore others have reported variable titers with different lots of FBS. Thus, OptiPEAK HEK293t can be used to overcome lot-to-lot variability observed with FBS. Overall, we have demonstrated that OptiPEAK HEK293t serum-free, ACF media can be readily implemented to manufacture high lentivirus titer.
References
- Laskowski, A. et al. (2019) Adherent HEK293t cells cultured in Pall’s iCELLis Bioreactor with OptiPEAK HEK293t blood-dree chemically defined medium exhibit robust and rapid population doubling times. Poster, ASGCT. https://invitria.com/resources/adherent-hek293t-cells-cultured-in-palls-icellis-bioreactor-with-optipeak-hek293t-blood-free-chemically-defined-media-exhibit-robust-and-rapid-population-doubling-times/
- Holic, N. et al. (2014) Influence of mildly acidic pH conditions on the production of lentiviral and retroviral vectors. Human Gene Therapy Clinical Dev., 25(3):178-85
| For additional product or technical information, please contact us at | |
| 2718 Industrial Drive
Junction City, KS 66441 |
Phone: 1.800.916.8311
Email: Info@InVitria.com |