- Home
- Recombinant Human Leukemia Inhibitory Factor for the Enhanced Expansion of Neural Stem Cells
Recombinant Human Leukemia Inhibitory Factor for the Enhanced Expansion of Neural Stem Cells
Published on 27 June 2019
Marcus Curl, Product Applications Scientist, InVitria
Workflow Summary
Applying neural stem cells (NSC) as a cell therapy has created new hope for the treatment of various neural degenerative diseases as well as the reversal of neural damage once thought to be irreparable. Currently, clinical trials are being performed to evaluate NSC for the treatment of stroke, Parkinson’s disease, spinal cord injuries, and many other indications [1]. As new potential applications emerge and new clinical trials begin, the need for scalable and safe sources of the foundational cell culture materials becomes essential to the future of this technology. The ability to quickly and reliably expand neural NSCs in culture has therefore been essential to the exploration of these novel, cell-based therapeutic approaches. The goal of this application note is to provide step-by-step instructions for using InVitria’s recombinant hLIF in the efficient, safe, and scalable expansion of NSC in culture.
Introduction
Applying neural stem cells (NSC) as a cell therapy has created new hope for the treatment of various neural degenerative diseases as well as the reversal of neural damage once thought to be irreparable. Currently, clinical trials are being performed to evaluate NSC for the treatment of stroke, Parkinson’s disease, spinal cord injuries, and many other indications [1]. As new potential applications emerge and new clinical trials begin, the need for scalable and safe sources of the foundational cell culture materials becomes essential to the future of this technology. The ability to quickly and reliably expand neural NSCs in culture has therefore been essential to the exploration of these novel, cell-based therapeutic approaches.
The yield and reproducibility of NSC expansion is improved when appropriate signaling molecules are introduced to the culture medium [2]. For example, the cytokine known as Leukemia Inhibitory Factor (LIF) has been shown to activate the LIF receptor on the surface of NSC, thereby enhancing the cells’ capacity for self-renewal [3]. LIF is therefore considered a powerful tool for improving expansion of NSC in vitro [2,3]. Currently, recombinant human (rhLIF) for cell-culture applications is predominantly biomanufactured using E. coli as the host organism [4] . While this protein-expression system has helped advance NSC technology, concerns exist about the safety and unintended effects of contamination from trace levels of bacterial endotoxin. Furthermore, different methods of production are required to support the manufacturing scale required for commercial development of stem cell therapies like NSC.
InVitria has improved both the scale and quality of rhLIF by biomanufacturing the cytokine in a eukaryotic but completely animal-free and non-mammalian host [5]. A side-by-side comparison of InVitria’s rhLIF and rhLIF isolated from E. coli for NSC expansion found that the two products produced equivalent decreases in doubling time. [6]. Furthermore, InVitria’s rhLIF product was found to contain 500-fold lower bacterial endotoxin compared to E. coli-derived rhLIF [5].
The goal of this application note is to provide step-by-step instructions for using InVitria’s recombinant hLIF in the efficient, safe, and scalable expansion of NSC in culture.
Materials Needed
Cells
- H9-embryonic-derived Neural Stem Cells (FisherScientific Prod#: N7800100)
Growth Media Preparation
- DMEM/F-12 (Sigma-Aldrich Prod#: D6429)
- L-glutamine (Sigma-Aldrich Prod#: G7513)
- rhLIF Animal Component Free (InVitria#: 777LIF048)
- rhFGF-2 (Sigma-Aldrich Prod#: F0291)
- rhEGF (Sigma-Aldrich Prod#: E9644)
- N-2 Media Supplement (ThermoFisher Prod#: 17502001)
Plate Design
- Corning™ BioCoat™ Poly-L-Ornithine/Laminin Multiwell Plate (FisherScientific Prod#: 354659)
- DPBS (Sigma-Aldrich Prod#: D8537)
- EDTA (Sigma-Aldrich Prod#:1233508)
- TrypLE Express Enzyme (FisherScientific Prod#: LS12604013)
Cell Counting
- Cell counter or hemocytometer
Equipment
- 37 °C Incubator at 5% CO2
Protocol:
Growth Media Preparation
- To create the standard background media formulation for NSC expansion, supplement L-glutamine into DMEM/F12 for a final concentration of 2mM, add N-2 supplement for a concentration of 1%, and finally add FGF-2 & EGF to the media at a concentration of 10 ng/ml each. This formulation will then be supplemented with different sources of recombinant hLIF to produce the experimental conditions.
- In separate media containers, supplement with recombinant hLIF. Add recombinant hLIF sourced from InVitria at a concentration 10 ng/ml.
Cell Plating & Expansion
- In Poly-L-ornithine/laminin-coated plates, plate cells at a concentration of 0.2 × 105viable cells/cm2 in growth media described above.
- Allow cells to grow in an incubator for 96-120 hours at 37 °C and 5% CO2.
- To harvest cells, add DPBS supplemented with TrypLE and 1 mM EDTA.
Cell Counting
- Determine the concentration of cells/ml using a hemocytometer or cell counter in order to calculate doubling time.
Results and Discussion:
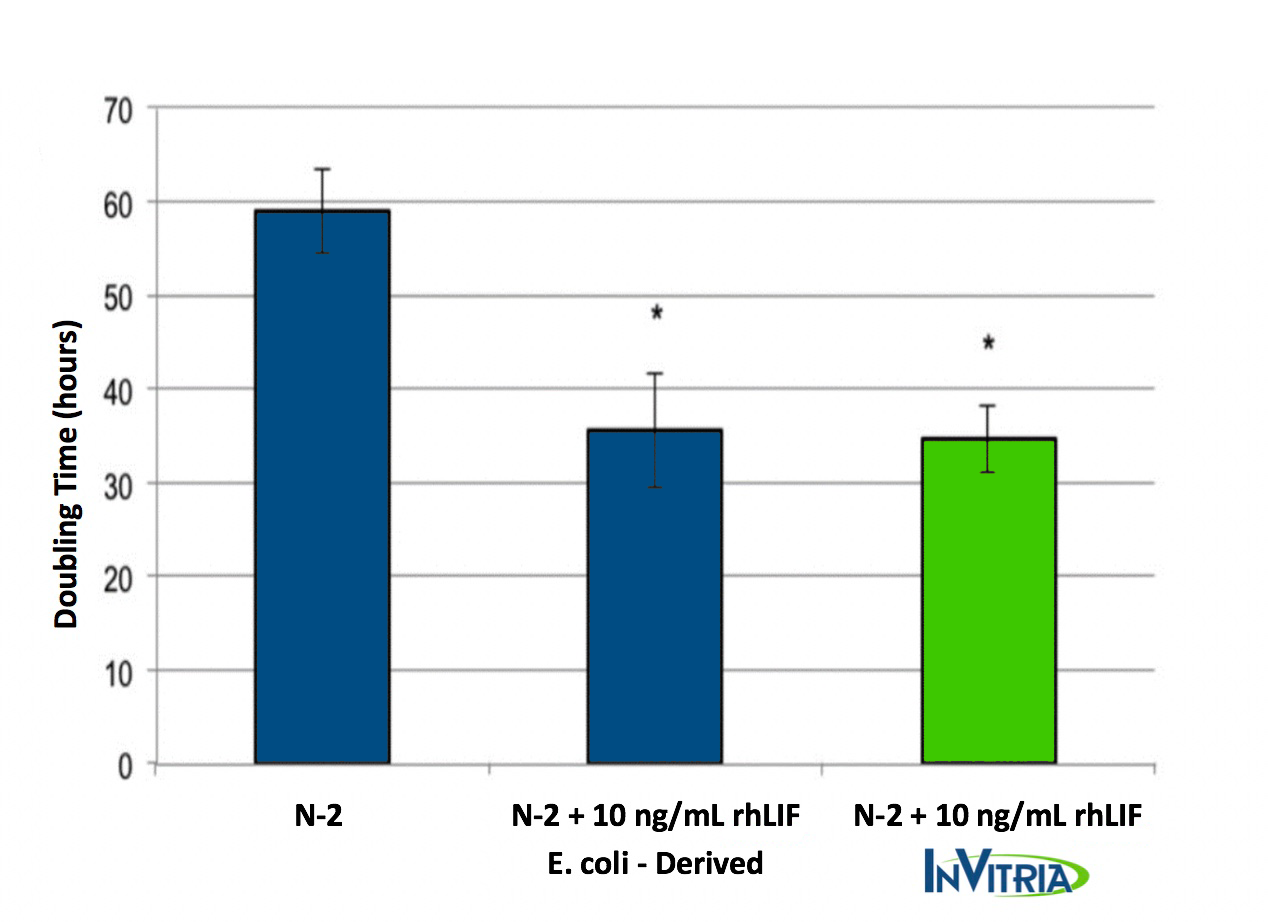
Supplementing the NSC growth medium with recombinant hLIF significantly decreased the doubling times for NSC expansion in vitro (Fig. 1). Additionally, no significant difference in growth performance was observed between recombinant rhLIF from InVitria and rhLIF derived from E. coli. These results suggest that InVitria’s non-mammalian expression system is capable of producing recombinant cytokines that are functionally comparable, yet more scalable and at higher quality required for clinical application.
With no change in performance, 500-fold lower levels of endotoxin, and unmatched scalability, InVitria’s rhLIF provides clear benefits over bacterial expression rLIF in the culture and expansion of NSC.
Footnotes
- Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. https://doi.org/10.1186/1741-7015-9-52
- Buono KD, Vadlamuri D, Gan Q, Levison SW. Leukemia inhibitory factor is essential for subventricular zone neural stem cell and progenitor homeostasis as revealed by a novel flow cytometric analysis. Dev Neurosci. 2012;34(5):449-462. https://doi.org/10.1159/000345155
- Pitman M, Emery B, Binder M, Wang S, Butzkueven H, Kilpatrick TJ. LIF receptor signaling modulates neural stem cell renewal. Mol Cell Neurosci. 2004;27(3):255-266. https://doi.org/10.1016/j.mcn.2004.07.004
- Gearing DP, Nicola NA, Metcalf D et al. Production of Leukemia Inhibitory Factor in Escherichia coli by a Novel Procedure and Its Use in Maintaining Embryonic Stem Cells in Culture. Bio/Technology. 1989;7(11):1157-1161. https://doi.org/10.1038/nbt1189-1157
- Youngblood BA, Alfano R, Pettit SC et al. Application of recombinant human leukemia inhibitory factor (LIF) produced in rice (Oryza sativa L.) for maintenance of mouse embryonic stem cells. J Biotechnol. 2014;172:67-72. https://doi.org/10.1016/j.jbiotec.2013.12.012
- Alfano R, Youngblood BA, Zhang D, Huang N, MacDonald CC. Human leukemia inhibitory factor produced by the ExpressTec method from rice (Oryza sativa L.) is active in human neural stem cells and mouse induced pluripotent stem cells. Bioengineered. 2014;5(3):180-185. https://doi.org/10.4161/bioe.28996