- Home
- Using Recombinant Transferrin in the Expansion of Hematopoietic Stem Cells Application Note
Using Recombinant Transferrin in the Expansion of Hematopoietic Stem Cells Application Note
Published on 29 May 2019
Marcus Curl, Product Applications Scientist, InVitria
A growing number of clinical cases have provided strong proof of concept that hematopoietic stem-cell (HSC) therapies are potential treatments for blood cancers and many other debilitating diseases that were once thought to be incurable [1]. A critical tool to enable the development of these therapies is expansion of HSCs in vitro [2]. However, supplementation of HSC growth media with serum-derived media supplements such as those included in xeno-free media and other animal-derived components can introduce adventitious pathogenic agents, create variability when cell expansion processes are scaled up for manufacturing, and can also present the risk of a supply chain interruption for clinical product manufacturing.
Introduction
A growing number of clinical cases have provided strong proof of concept that hematopoietic stem-cell (HSC) therapies can be potential treatments of blood cancers and many other debilitating diseases that were once thought to be incurable [1]. A critical tool to enable development of these therapies is expansion of HSCs in vitro [2]. However, supplementation of HSC growth media with serum-derived media supplements such as those included in xeno-free media and other animal-derived components can introduce adventitious pathogenic agents, create variability when cell expansion processes are scaled up for manufacturing, and can also present the risk of a supply chain interruption for clinical product manufacturing.
Using strictly chemically defined media, supplemented with alternatives to serum or animal-derived components has repeatedly been identified as a viable strategy for reducing variation, improving consistency, and enabling scalability for cell therapies [3,4]. As the trend toward the use of chemically defined media formulations increases, an important consideration is the need to achieve effective intracellular iron delivery. Iron is a critical aspect of cellular metabolism, cell replication, and other specialized functions [5]. However, despite the central role of iron in cell biology, free, ferrous iron (Fe2+ ) is readily oxidized in solution to its ferric form (Fe3+). Ferric iron is a catalyst for the formation of reactive oxygen species and free radicals that can be toxic in living animals and in cell culture systems [5,6].
Transferrins are iron-binding glycoproteins, produced by the liver, that occur naturally in blood serum. As seen in Figure 1, their function is to recycle iron to maintain essential cellular processes while suppressing the opportunistic formation of harmful molecules. Transferrin controls the level of free, circulating iron in the body by binding to extracellular free ferric iron atoms and delivering them into cells through endocytosis [7]. Once transferrin delivers its iron payload within the cell it is recirculated as iron-free transferrin and available to bind additional iron in a catalytic cycle. This cycle occurs every 15 minutes in rapidly dividing cells.

By sequestering iron, transferrin makes it available for cellular functions while significantly reducing the capability of circulating free iron to catalyze the formation of toxic free radical molecules [6]. In cell culture, transferrin is customarily supplied as a component of fetal bovine serum; however, chemically defined media require a purified, recombinant form of this essential component because they do not contain serum.
Optiferrin is a recombinant and completely animal-free form of human transferrin, manufactured at large scale by InVitria using a non-mammalian host. It is therefore an optimal source of transferrin supplementation for chemically defined culture media for the expansion of HSCs and other sensitive stem-cell applications. Optiferrin serves as an iron chaperone for in vitro cell culture. This product is an essential tool for enabling the development of more robust, top-performing chemically defined media without the risk of iron catalyzed free-radical formation, oxidation reactions, adventitious pathogens, or animal- or serum-derived contaminants.
Materials Needed:
Optiferrin Reconstitution
- Optiferrin (InVitria Prod#: 777TRF029)
- Basal media of choice or Phosphate Buffered Saline (PBS)
- Analytical Balance
- Sterile Container – PETG Diagnostic Bottle: Sterile (Fischer Scientific #02-925-104)
Stock Solution Filtration
- PES Low Protein Binding Membrane Filter, 0.2 Micron, 30mm (Celltreat #229747)
- Syringe: Sterile Luer-Lock Syringe, 3 mL (Medline #SYR103010H)
Protocol:
200mg/ml Stock Solution Preparation
- For 5 to 10 ml final volume of 200mg/ml stock solution, weigh out 1 to 2g of Optiferrin into a sterile container, using an analytical
- Calculate reconstitution volume using the following equation:

- Slowly add 70% of the final volume of PBS or basal media, taking care to prevent the production of
- Place the bottle in a 2 to 8 °C water bath until all protein has
- Measure the new volume after all Optiferrin has dissolved and add the remaining volume of PBS or basal media to equal the calculated resuspension
- Pipet up and down gently to ensure even
Stock Solution Filtration
- In a sterile laminar-flow hood, pour the reconstituted Optiferrin stock solution into a sterile, Luer-lock syringe.
- Attach an0.2-micron PES filter.
- Filter solution into a new, sterile container
Supplementation Level Determination and Addition to Cell Culture Medium
- Experimentally determine the optimal concentration range for Optiferrin supplementation by titrating the stock solution. Based on the cell-specific supplementation level determined, add the corresponding volume of the 200mg/ml stock solution to the growth media.

In the case of HSC, for a theoretical supplementation level of 50mg/L, add 0.25ml of the 200mg/ml stock solution of Optiferrin to 1L of media to produce a final concentration of 50mg/L in the media formulation.
Results and Discussion:
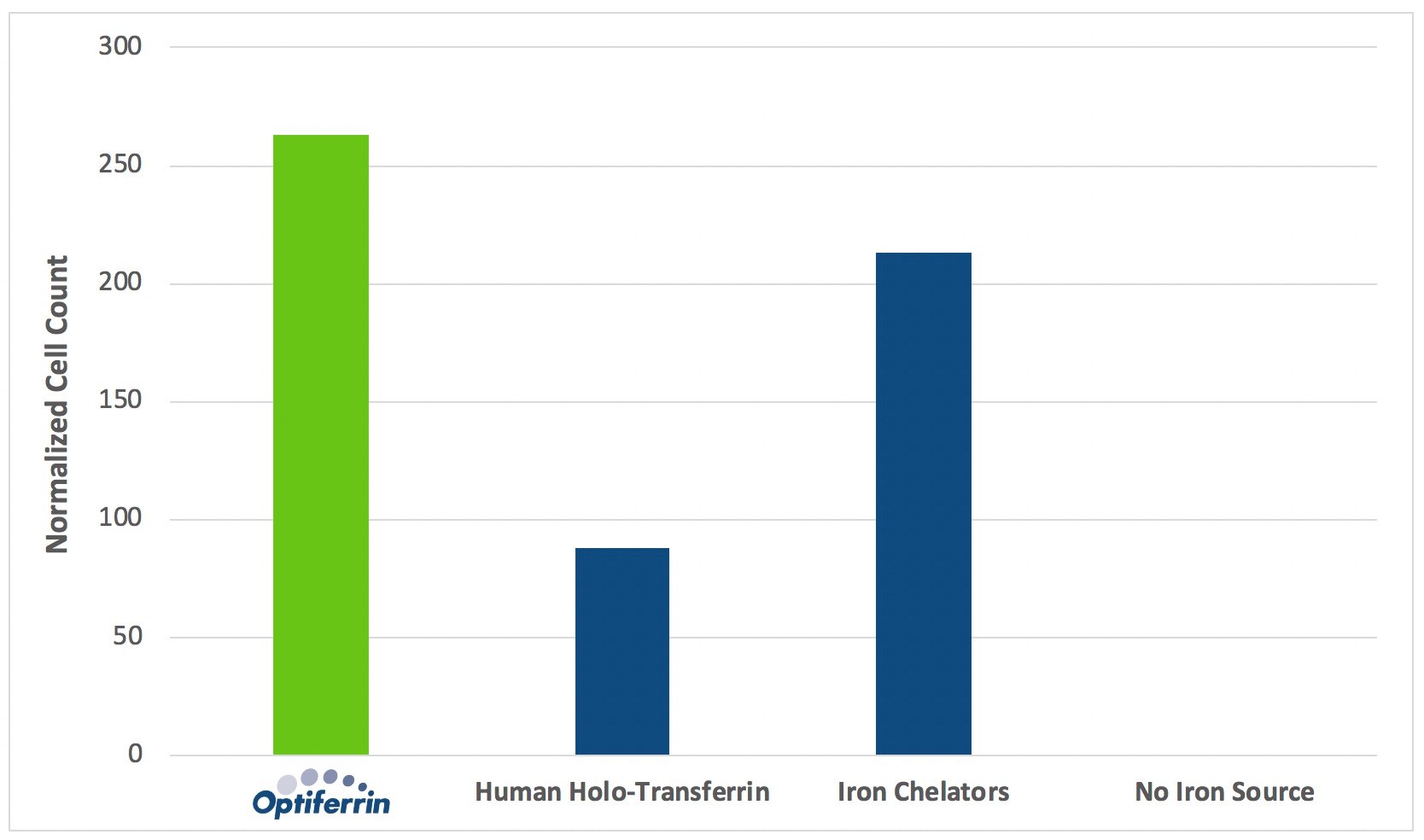
As seen in Figure 2, the presence of iron carrier and iron chelator supplementation influences proliferation rates of human HSC. Compared to culture conditions lacking iron chelators or iron carriers, supplementation with these components provides significant benefit to the proliferation of human HSC. Over a 6-day expansion period, recombinant human transferrin, Optiferrin, outperformed human transferrin sourced from serum by nearly threefold, and also yielded superior performance compared to iron chelators. These results support the use of Optiferrin as a supplement to improve proliferation of human HSC in applications requiring chemically defined culture media.
Footnotes
- Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. https://doi.org/10.1186/1741-7015-9-52
- Park B, Yoo KH, Kim C. Hematopoietic stem cell expansion and generation: the ways to make a breakthrough. Blood Res. 2015;50(4):194-203. https://doi.org/10.5045/br.2015.50.4.194
- Chisini LA, Conde MCM, Grazioli G et al. Venous Blood Derivatives as FBS-Substitutes for Mesenchymal Stem Cells: A Systematic Scoping Review. Braz Dent J. 2017;28(6):657-668. https://doi.org/10.1590/0103-6440201701646
- van der Valk J, Brunner D, De Smet K et al. Optimization of chemically defined cell culture media–replacing fetal bovine serum in mammalian in vitro methods. Toxicol In Vitro. 2010;24(4):1053-1063. https://doi.org/10.1016/j.tiv.2010.03.016
- Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434(3):365-381. https://doi.org/10.1042/BJ20101825
- Baldwin DA, Jenny ER, Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984;259(21):13391-13394.
- Luck AN, Mason AB. Transferrin-mediated cellular iron delivery. Curr Top Membr. 2012;69:3-35. https://doi.org/10.1016/B978-0-12-394390-3.00001-X