- Home
- Reducing Serum-Associated Contaminants from Growth Media in Primary Cell Culture
Reducing Serum-Associated Contaminants from Growth Media in Primary Cell Culture
Published on 4 March 2019
Atherly Pennybaker, Media Formulation and Product Applications Specialist, InVitria
Workflow Summary
Supplement basal media with ITSE + A to a final 1x-2x concentration. Reduce typical percentage of added FBS by 50-90%. Expand cells as normal in ITSE + A-supplemented media for desired expansion time. Perform post-expansion cell count and doubling-time calculation.
Introduction
Fetal bovine serum contains an abundance of critical proteins and components that are crucial for promoting optimal cellular proliferation in ex vivo applications. For example, insulin promotes uptake of glucose and amino acids. Transferrin delivers iron into cells via the transferrin receptor to drive cellular respiration. And albumin—the most abundant serum protein—delivers lipids for energy, has high antioxidant capacity, and binds to toxins, among other functions [1-4]. Other critical serum components include selenium, required for activity of various antioxidant enzymes, and ethanolamine, which is a phospholipid precursor.
However, increasing recognition of the risks of unwanted contaminants in serum sourced from animals has driven a push to reduce or eliminate the use of serum in primary cell-culture systems. To avoid loss of cell-culture performance associated with reducing or eliminating serum, it is necessary to add a defined supplement containing recombinant versions of the critical serum components discussed above. To address the functionality of growth media after serum reduction or replacement, InVitria has created a defined supplement containing the recombinant serum proteins insulin, transferrin, and albumin plus selenium and ethanolamine, incorporated in a convenient liquid concentrate called “ITSE + A”. This supplement contains all of the critical serum components in one solution. All of the protein components are synthetically manufactured using recombinant DNA technology in an animal-free host. This product enables the reduction of serum and its associated contaminants from primary cell culture and leading to improved ex vivo cellular analyses.
Materials Needed
Growth Media Preparation
- Basal growth media of choice
- ITSE + A, 100x supplement (product no. 777ITS092)
- Fetal bovine serum
- Penicillin/streptomycin (optional)
Cells
- Tumor cell lines
- Primary cell lines
Cell Expansion & Subculturing
- Plates and/or flasks
- Dissociation solution (for adherent cell types only)
- Centrifuge
- Cell counter/hemocytometer
Protocol
Growth Media Preparation
- Supplement ITSE + A into basal medium to a final 1x-2x concentration: For 1 liter of complete medium, add 10 mL of the 100x concentrated ITSE + A for a 1x final concentration and 20 mL of concentrated 100x ITSE + A for 2x final concentration.
- Supplement with fetal bovine serum (FBS) at a reduced percentage. Typical inclusion levels reported with ITSE + A range between 1% and 5% FBS final concentration, though this is dependent on the cell type and the lot of FBS being used.
- If desired, add penicillin/streptomycin at 0.1x-0.5x final concentration.
Cells
- Acquire desired cell type from ATCC or similar cell bank.
- Thaw cells in recommended growth media containing 10% FBS and allow cells to grow until confluence has been achieved.
Cell Expansion and Subculturing
- Harvest thawed cells from growth surface, if applicable, using 0.25% trypsin followed by a 1 in 10 washout dilution in growth media. If cells are suspension-type, collect the entire cell suspension.
- Pellet cells by centrifugation and resuspend cells in the reduced-serum growth media supplemented with ITSE + A.
- Subculture cells for standard subculture times following routine protocols and passage in the same reduced-serum growth media containing ITSE + A.
- At each passage, harvest cells, take cell counts and seed subsequent passages at desired cell density. Calculate doubling time as follows:
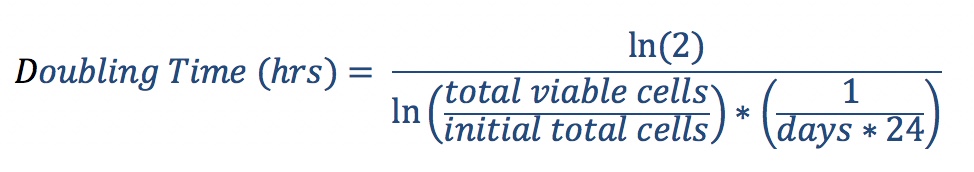
Results and Discussion
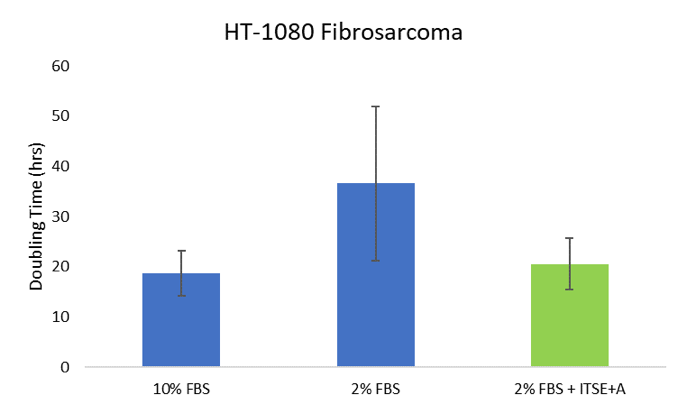
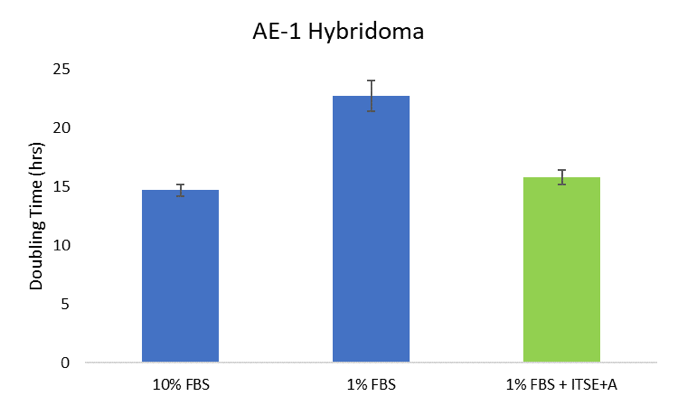
Reduced-serum conditions were tested for culture of fibrosarcoma and hybridoma cells in comparison to standard 10% FBS growth media. As shown by increased doubling time (i.e. slower growth), fibrosarcoma cells were sensitive to an 80% reduction in FBS; however, when ITSE + A was added to the reduced-serum media, their performance was comparable to cells cultured in 10% FBS (Figure 1). Similarly, hybridoma cells were able to proliferate even when serum was reduce by 90% when media was supplemented with ITSE + A (Figure 2). Combined, these results show that the addition of ITSE + A enables significant reduction in serum supplementation, for multiple cell types. By replacing the most well-known serum proteins and components with defined, recombinant sources, cell viability and proliferation can be maintained in reduced-serum cell culture applications.
Footnotes
- Murakami, H., Masui, H., Sato, G. H., Sueoka, N., Chow, T.P., Kano-Sueoka, T. Growth of hybridoma cells in serum-free medium: Ethanolamine is an essential component. 1982. Proc. Natl. Acad. Sci. USA. 79:1158-1162.
- Czech, M.P. Molecular mechanism of insulin action. 1977. Ann. Rev. Biochem. 46:359-384.
- Aisen, P. Iron in Biochemistry and Medicine, ed. Jacobs. A. and Worwood, M., Academic Press, New York, pp.87-129 (1980).
- Saito, Y., Yoshida, Y., Akazawa, T., Takahashi, K., Niki, E. Cell death caused by selenium deficiency and protective effect of antioxidants. 2003. J. Biol. Chem. 278(41):39428-34.