- Home
- Scaling Innovation: How DiscGenics and InVitria are Leading the Future of Cell Therapy
Scaling Innovation: How DiscGenics and InVitria are Leading the Future of Cell Therapy
Published on 11 October 2024
Leveraging animal-free materials and scalable manufacturing processes to set new standards in biomanufacturing and cell therapy development.
Reading time: 5 minutes
Share this article:
The cell therapy industry stands on the edge of a transformative era. With advancements in treatment options and scalable manufacturing processes, companies like DiscGenics™ are leading the charge, delivering innovative therapies for previously intractable conditions. Simultaneously, market dynamics are shifting, with renewed investor confidence signaling a brighter future for the sector.
Through strategic partnerships, such as the collaboration between DiscGenics and InVitria®, these breakthroughs are not only becoming more accessible to patients but are setting new standards for safety, scalability, and efficiency in biomanufacturing. Together, they exemplify what’s possible when innovation meets meticulous process control.
DiscGenics: A Trailblazer in Allogeneic Cell Therapy
![]()

Millions of patients worldwide suffer from lumbar disc degeneration, a condition often leading to chronic pain, reduced mobility, and opioid dependency. For these individuals, treatment options have been limited to invasive surgeries or lifelong pain management. DiscGenics has disrupted this narrative with its allogeneic disc progenitor cell therapy, which has shown remarkable promise in increasing disc volume, reducing pain, and improving the quality of life for patients in clinical trials.
DiscGenics’ success is built on their remarkable ability to transition from small-scale, bench-top procedures to a fully scalable manufacturing process, enabling the production of thousands of doses. This achievement is best captured in the words of Jeff Zurawski, PhD, Associate Director of Bioprocess Development at DiscGenics:
“DiscGenics has developed a commercially viable manufacturing process for their allogeneic disc cell therapy capable of producing thousands of doses. The new scalable manufacturing process streamlines process development and increases raw material control and compliance, eliminating human-derived components.”
This focus on scalability not only ensures broader access to the therapy but also sets the stage for long-term commercial success. The ability to scale effectively is becoming a defining factor in the cell therapy industry, separating those with a viable path to market from those stuck in the lab.
InVitria: Pioneering Safe, Scalable Biomanufacturing
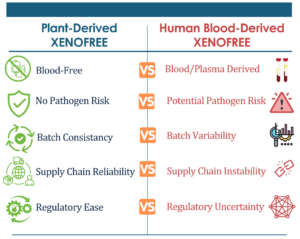
One of the key elements in DiscGenics’ success is its strategic use of InVitria’s animal-free, recombinant materials. By incorporating products like Optiferrin® and Exbumin®, DiscGenics was able to eliminate human-derived components from its manufacturing process. This not only reduces variability and enhances safety but also positions DiscGenics to meet the stringent regulatory requirements of late-stage clinical trials.
As the cell therapy sector becomes increasingly focused on reducing risks, it’s important to address a widespread misconception around the use of Xeno-Free products. Many companies have marketed “Xeno-Free” solutions, claiming that they are safer by eliminating animal-derived materials. However, Xeno-Free does not mean risk-free. In fact, Xeno-Free transferrin, which is sourced from human materials, introduces a different and potentially more dangerous risk—human pathogens.
A pathogen that contaminates Xeno-Free transferrin is, by definition, a human pathogen, which could pose significant risks in biomanufacturing and clinical applications. While “Xeno-Free” may sound appealing, it is a very risky option compared to InVitria’s animal-free recombinant materials, which eliminate both animal and human contamination risks. InVitria’s solutions allow manufacturers to maintain a higher standard of safety, removing the variability that can come with human-derived materials.
A Market Poised for Growth
The cell and gene therapy industry has faced its share of challenges in recent years. The pandemic-driven investment boom of 2020 and 2021 gave way to more cautious funding in 2022 and 2023, as capital became harder to secure, particularly for early-stage companies. However, signs of recovery are emerging.
According to Tim Hunt, CEO of the Alliance for Regenerative Medicine, $10.9 billion was invested in the first half of 2024, already surpassing the total for 2019. While this is still a far cry from the highs of 2021, it reflects renewed confidence in the sector. Much of this investment has been directed towards later-stage companies with advanced clinical trials and established data, like DiscGenics.
For companies like DiscGenics, this is a promising sign. Investors are seeking opportunities in cell therapies that are ready to scale and have demonstrated potential in improving patient outcomes. With their scalable manufacturing process and late-stage clinical progress, DiscGenics is well-positioned to benefit from this renewed flow of capital.
The Importance of Scalability, Process Control, and Raw Material Selection
As DiscGenics’ success demonstrates, scaling up a manufacturing process is not just about increasing production capacity—it’s about ensuring that the therapy remains consistent, safe, and compliant as it moves through clinical trials and into commercialization. This is where process control and raw material quality become essential.

DiscGenics has embraced dynamic culture systems, moving away from flask-based, manual processes to stirred tank bioreactors that allow for greater control and scalability. This shift is a testament to the company’s forward-thinking approach, positioning them to meet the demands of large-scale production without compromising quality.
Moreover, by integrating InVitria’s recombinant products early in their development process, DiscGenics has been able to mitigate the risks associated with human-derived components, ensuring that their therapy is both safe and consistent from batch to batch. This attention to quality and scalability is what will allow DiscGenics to bring its therapies to a broader market while maintaining the highest standards of care.
Looking Forward: The Road to Commercial Success
As the cell and gene therapy sector regains momentum, companies that have invested in scalable, high-quality manufacturing processes are poised for success. The collaboration between DiscGenics and InVitria serves as a blueprint for how strategic partnerships can help navigate the challenges of biomanufacturing and bring transformative therapies to market.
The message is clear: scalability, process control, and quality will define the next wave of success in the cell therapy space. Companies that prioritize these factors from the outset will not only secure investment but also deliver the life-changing therapies that patients around the world desperately need.
Want to learn more about DiscGenics? Visit their website at www.discgenics.com
Ready to scale your cell therapy manufacturing process? Contact us or call 800-916-8311 to learn how InVitria’s animal-free, recombinant solutions can help you achieve the consistency, safety, and scalability necessary for clinical and commercial success.
Featured Products
 Optiferrin® – Recombinant Human Transferrin
Optiferrin® – Recombinant Human Transferrin
Optiferrin® is an animal-free recombinant human transferrin, designed to improve cell culture performance with consistent iron delivery. It eliminates contamination risks associated with human-derived transferrin and is key to scalable biomanufacturing processes like those at DiscGenics.
 Exbumin® – Recombinant Human Albumin
Exbumin® – Recombinant Human Albumin
Exbumin® is a recombinant human albumin that replaces animal- and human-derived albumin, ensuring greater consistency and safety in biomanufacturing. It helps reduce variability and contamination risks, making it ideal for cell therapy production.
 Cellastim S® – Recombinant Human Albumin
Cellastim S® – Recombinant Human Albumin
Cellastim S® is an animal-free recombinant human albumin optimized for cell culture applications. It enhances cell growth, survival, and productivity in a wide range of biomanufacturing processes, helping companies like DiscGenics scale their therapies with greater control and consistency.
Footnotes
References
- Alliance for Regenerative Medicine. (2024). Investment in cell and gene therapy ticks up after a challenging period. BioSpace. https://www.biospace.com/business/big-pharmas-interest-grows-in-evolving-cell-and-gene-therapy-sector
- DiscGenics, Inc. (2024). DiscGenics develops scalable allogeneic disc progenitor cell therapy process. ARM Mesa 2024 Presentation.
- Hunt, T. (2024, October 8). Welcome remarks at 2024 Cell & Gene Meeting on the Mesa. Presented at the Alliance for Regenerative Medicine’s Cell & Gene Meeting on the Mesa, Phoenix, AZ.
- Zurawski, J. (2024). DiscGenics: Bioprocess development and manufacturing scalability. Presented at the 2024 Cell & Gene Meeting on the Mesa.
Copyright © 2024 InVitria, Inc. All rights reserved.
Reproduction or distribution of any InVitria materials, in whole or in part, is prohibited without prior written consent. All logos, names, designs, and marks displayed, including InVitria®, and the InVitria® brand design, are trademarks or service marks owned or licensed by InVitria, Inc., Kansas, USA, unless otherwise noted. For details on InVitria’s registered intellectual property, patents, and additional terms and conditions, please visit www.InVitria.com/terms.