- Home
- Variability and Failed Lots: The Hidden Quality Control Challenge of Serum & Serum-Derived Products
Variability and Failed Lots: The Hidden Quality Control Challenge of Serum & Serum-Derived Products
Published on 6 January 2026
Marcus Curl, VP of Product Applications
Reading time: 4 minutes
Share this article:

The biopharmaceutical industry faces a critical quality control challenge: the inherent variability of serum and plasma-derived products. While traditionally relied upon for cell culture, blood-derived albumin introduces significant lot-to-lot inconsistencies in protein heterogeneity, growth factor content, and biochemical profiles. These variations directly impact manufacturing success, particularly in sensitive applications like CAR-T cell therapy, where they compound patient-specific variability and increase the risk of batch failure. Furthermore, relying on variable raw materials necessitates extensive comparability studies and complex process qualification to meet stringent regulatory requirements. As agencies increasingly favor animal-origin-free (AOF) and chemically defined solutions, transitioning to recombinant alternatives is no longer just a safety preference but a strategic necessity for manufacturing consistency and regulatory speed.
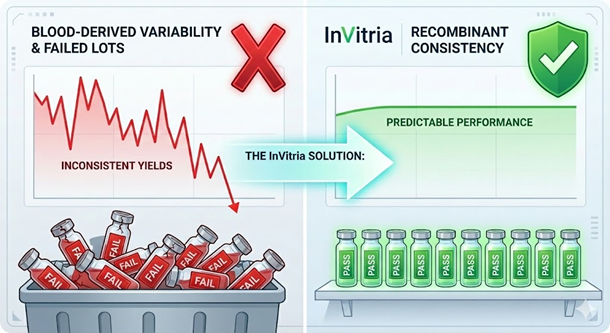
Lot-to-Lot Variability in Serum Albumin
Even when sourced from reputable suppliers, blood-derived albumin exhibits inherent variability due to its biological origin. This variability arises in part from protein heterogeneity. Plasma-derived human serum albumin is not a single, uniform molecular species but a complex mixture of protein variants that differ in post-translational modifications, oxidation state, and bound ligands. Studies using reverse phase HPLC and mass spectrometry have revealed:
Protein Heterogeneity and Modifications
- Multiple peaks and species in plasma-derived albumin compared to recombinant alternatives (Cell & Gene Therapy Insights, 2018)
- Oxidation and glycation products that vary between donors and collection conditions (Fanali et al., 2021)
- Bound fatty acids, metal ions, and small molecules that differ by lot and can affect cell culture performance (Fanali et al., 2021)
Research comparing plasma-derived albumin to recombinant alternatives found that while recombinant albumin “resembled serum-derived albumin,” the recombinant product exhibited a “more consistent profile” (Cell & Gene Therapy Insights, 2018) . This consistency translates to improved reproducibility in cell expansion and downstream processing.
Source-Dependent Composition Differences
A study characterizing human AB serum from different plasma sources (plasma removed from whole blood after 24 hours of collection versus plasma cryoprecipitate reduced) found variations in biochemical profiles (Chieregato et al., 2016). While all produced batches met quality standards, differences were observed in:
- Glucose and calcium levels affected by the volume of additives used during plasma-to-serum conversion
- Growth factor content varying with donor populations and collection methods
- pH changes during storage affecting product stability over time
The study concluded that using blood bank-derived plasma provides better lot-to-lot consistency due to careful donor screening but acknowledged that “some differences were observed” between sources and over storage time (Chieregato et al., 2016).
Cross-Species Variability
For applications using bovine serum albumin (BSA) or fetal bovine serum, variability is even more pronounced. Natural sequence variation in serum albumins from different species demonstrates:
- Inherent sequence variations between BSA, HSA, and rat serum albumin (RSA) affecting protein structure
- Different conformational stabilities with BSA and HSA existing as monomers while RSA forms multimers
- Unique adsorption characteristics and protein layer formation affecting cell-surface interactions
These structural differences translate to functional variability in cell culture applications, making it difficult to achieve consistent performance when switching between lots or suppliers (Latour et al., 2020, He et al., 2011).
Variability is amplified when bovine serum albumin or fetal bovine serum is used. Natural sequence variation between bovine, human, and rat serum albumins results in differences in protein structure, conformational stability, and adsorption behavior at interfaces (Understanding how natural sequence variation in serum albumin affects protein adsorption, 2020). These structural differences translate into functional variability in cell culture, complicating reproducibility when switching lots or suppliers (Large-scale production of functional human serum albumin from transgenic rice seeds, 2011).
Impact on Manufacturing: Batch Failures and Process Drift
Lot-to-lot variability in serum and serum-derived products creates tangible manufacturing challenges:
Failed CAR-T Manufacturing Lots In CAR-T cell manufacturing, “inherent variability between the sources of patients’ cells is a key reason for the higher batch failure seen with CAR-T cell therapies” (Cell & Gene Therapy Insights, 2017). When this inherent patient-to-patient variability is compounded by variable raw materials like human AB serum, the risk of batch failure increases significantly.
The FDA guidance for CAR-T cell products emphasizes that “CAR T cell manufacturing involves multiple biological materials and complex multi-step procedures, which are potential sources of variability among product lots” (U.S. Food and Drug Administration [FDA], 2022). Control of the manufacturing process and appropriate in-process and lot release testing are crucial to ensure consistent Critical Quality Attributes (CQAs) (FDA, 2022).
Comparability Studies and Change Control
When manufacturers identify performance issues with a particular lot of serum or need to change suppliers, they face regulatory requirements for comparability studies (Salt, 2021). These studies must establish that:
- Raw material changes have not altered the final product
- Cell growth, viability, and phenotype remain consistent
- Potency and safety profiles are maintained
Early adoption of serum-free, chemically defined media eliminates the need for these comparability studies, reducing development timelines, and regulatory complexity. As one industry expert noted, removing serum “removes the need for comparability studies to establish that any raw material changes have not altered the final product” (Salt, 2021).
Process Qualification Challenges Establishing Critical Process Parameters (CPPs) requires understanding how raw material variability affects product CQAs (FDA, 2022). With variable serum lots, manufacturers face:
- Wider acceptable ranges for process parameters to accommodate raw material variability (FDA, 2022)
- More extensive process qualification studies to characterize the impact of different lots (FDA, 2022)
- Ongoing monitoring and trending to detect lot-to-lot effects on product quality (FDA, 2022)
Chemically defined, recombinant alternatives simplify process qualification by removing a major source of variability (Salt, 2021, InVitria, 2025a, Rathore et al., 2022).
Regulatory Perspectives on Serum-Free Manufacturing
Regulatory agencies increasingly emphasize the use of safer, well-characterized raw materials in biologics development and manufacturing. FDA guidance on the characterization and qualification of biological materials underscores the agency’s expectation that manufacturers identify, assess, and control risks associated with blood- and animal-derived components, including variability and adventitious agent contamination (U.S. Food and Drug Administration, 2010). More recent FDA guidance further reinforces the need for comprehensive risk assessment and justification when human- or animal-derived materials are used in cell and gene therapy manufacturing (U.S. Food and Drug Administration, 2024).
Collectively, these guidances reflect regulatory expectations that favor rigorous material characterization, traceability, and risk mitigation, which align with the broader industry shift toward chemically defined and animal-origin-free raw materials.
Key regulatory considerations highlighted in FDA guidance include:
- Risk-based qualification of biological materials, including evaluation of contamination potential, variability, and suitability for intended use (U.S. Food and Drug Administration, 2010, 2024)
- Heightened scrutiny of human- and animal-derived components, requiring detailed documentation and justification when such materials are incorporated into manufacturing processes (U.S. Food and Drug Administration, 2024)
- Emphasis on consistency and control of raw materials to support product quality, safety, and regulatory compliance across development and manufacturing stages (U.S. Food and Drug Administration, 2010)
EMA guidance, including ICH Q5A(R2), establishes a risk-based framework for viral safety evaluation that discourages unnecessary reliance on animal-derived raw materials and supports the use of safer alternatives to minimize contamination risk (International Council for Harmonisation, 2023; European Medicines Agency, 2018).
Solving the Variability Crisis
The data is clear: the biological “noise” inherent in serum-derived products is a primary driver of process drift and manufacturing failure. To achieve the level of precision required for next-generation therapies, the industry must move beyond the unpredictable nature of plasma-derived supplements.
InVitria’s product offerings provide the definitive solution to this challenge. By leveraging a highly controlled, non-mammalian expression system, InVitria produces recombinant human serum proteins and media supplements that are chemically defined and 100% animal-origin-free. Unlike plasma-derived alternatives that fluctuate with donor health and collection methods, InVitria’s products offer:
- Absolute Consistency: Eliminating the heterogeneity of blood-derived lots to ensure every batch of media performs identically.
- Regulatory Streamlining: Reducing the documentation burden and eliminating the need for costly comparability studies required by raw material changes.
- Enhanced Performance: Optimized for high-growth applications, providing the stability and purity necessary to maximize yields in cell and gene therapy manufacturing.
By integrating InVitria’s technology, manufacturers can replace biological uncertainty with predictable, scalable, and high-quality results, ensuring that the next generation of life-saving medicines is built on a foundation of total process control.
The following content is gated. Please, subscribe to open access to it.
Footnotes
References
Cell & Gene Therapy Insights. (2018). Utilization of recombinant albumins in the expansion of T lymphocytes.
https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/326/Utilization-of-Recombinant-Albumins-in-the-Expansion-of-Human-T-Lymphocytes
Fanali, G., di Masi, A., Trezza, V., Marino, M., Fasano, M., & Ascenzi, P. (2021). Structural and biochemical features of human serum albumin. International Journal of Molecular Sciences, 22(5), 2560. https://pmc.ncbi.nlm.nih.gov/articles/PMC8395139/
Chieregato, K., et al. (2016). Characterization of human AB serum for mesenchymal stem cell expansion. PLoS ONE, 11(5), e0155612.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4863213/
Latour, R. A., et al. (2020). Understanding how natural sequence variation in serum albumin affects protein adsorption. Colloids and Surfaces B: Biointerfaces, 193, 111123.
https://www.sciencedirect.com/science/article/pii/S0927776520303276
He, Y., et al. (2011). Large-scale production of functional human serum albumin from transgenic rice seeds. Proceedings of the National Academy of Sciences, 108(47), 19078–19083. https://www.pnas.org/doi/10.1073/pnas.1109736108
Cell & Gene Therapy Insights. (2017). The regulation of CAR-T cells.
https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/88/The-Regulation-of-CAR-T-Cells
U.S. Food and Drug Administration. (2010). Guidance for industry: Characterization and qualification of cell substrates and other biological materials used in the production of viral vaccines for infectious disease indications. https://www.fda.gov/media/78428/download
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. (2023). ICH guideline Q5A(R2): Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin. https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q5ar2-guideline-viral-safety-evaluation-biotechnology-products-derived-cell-lines-human-or-animal-origin-step-5_en.pdf
European Medicines Agency. (2018). Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products.
https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-clinical-aspects-gene-therapy-medicinal-products_en.pdf
Food and Drug Administration. (2022). Considerations for the development of chimeric antigen receptor (CAR) T cell products.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-development-chimeric-antigen-receptor-car-t-cell-products
Salt, S. (2021, October 12). Technology digest: The importance of serum- and animal component-free media for stem cell-based therapeutics. RegMedNet. https://www.regmednet.com/technology-digest-the-importance-of-serum-and-animal-component-free-media-for-stem-cell-based-therapeutics/
InVitria. (2025a). How regulatory bodies are driving the shift to animal-origin-free solutions. https://invitria.com/resources/regulatory-bodies-shift-animal-origin-free-solutions/
Huang, N., et al. (2011). Large-scale production of functional human serum albumin from transgenic rice seeds. Proceedings of the National Academy of Sciences, 108(7), 2848–2853.
https://www.pnas.org/doi/10.1073/pnas.1018286108
Cell Culture Dish. (2020). FDA issues guidance for warning labels on all drugs produced using blood products. https://cellculturedish.com/fda-issues-guidance-for-warning-labels-on-all-drugs-produced-using-blood-products-including-plasma-derived-albumin/
InVitria. (2025b). Why border disruptions threaten blood-derived raw materials and what biomanufacturers can do about it.https://invitria.com/resources/why-border-disruptions-threaten-blood-derived-raw-materials-and-what-biomanufacturers-can-do-about-it/