- Home
- VERO Expansion in the iCellis® Bioreactor with Chemically Defined and Blood Free Virus Production Media
VERO Expansion in the iCellis® Bioreactor with Chemically Defined and Blood Free Virus Production Media
Published on 25 January 2023
Introduction
Manufacturing vaccines for both preventative and therapeutic applications requires the production of large amounts of virus from host cell substrates. Increased demand for these viral products has induced a major shift in the model of viral vaccine manufacturing from more primitive platforms, such as embryonated chicken eggs (ECE), to more robust large-scale methods that utilize continuous cell line (CCL)-based systems in 3D cell culture systems [1]. VERO cells, derived from the kidney epithelial tissue of an African monkey, have become the most widely used and best characterized cell line for vaccine production and have now been utilized as the manufacturing platform for numerous types of human vaccines for nearly 40 years [2].
Traditionally, VERO cells are expanded in Dulbecco’s Modified Eagles Medium (DMEM) or Eagles Minimum Essential Medium (EMEM) containing 10% fetal bovine serum (FBS) (EMEM-10%FBS) and have successfully produced a multitude of virus types for vaccine production at commercially viable levels [2] [3] [4]. However, due to safety concerns of potential adventitious agents presented in FBS, poor reproducibility, and limited availability for supply, the guidance of regulatory agencies indicates that alternatives to bovine serum should be adopted in vaccine production [5] [6].
VERO cells can be readily adapted to serum-free conditions, provided that the critical serum protein components are replaced to support the necessary biological functions these cells depend on for successful proliferation. OptiVERO®, a chemically defined media that incorporates recombinant versions of serum proteins required for VERO growth, has demonstrated efficacy in expanding VERO cells and subsequently producing virus in both flask and microcarrier culture [7]. Therefore, this serum free media represents an advancement for virus production in that VERO cells can be expanded in the absence of poorly defined serum-containing media.
Another advancement that has been rapidly adopted as the leading workhorse for clinical viral vector and vaccine manufacturing is the Pall iCELLis® Bioreactor. As a single-use, fixed-bed bioreactor, the heart of the iCELLis® technology is the unique compact microfibers that make up the fixed bed and provide a large growth surface area to increase volumetric productivity over traditional stirred tank reactors. With its dynamic environment and fully automated PID control system, the entire cell culture process from seed train to final product is simplified and readily adaptable from R&D to clinical manufacturing.
Here, the novel OptiVERO® chemically defined media is combined with the iCELLis® platform to determine the efficacy of these two innovations to viral vaccine manufacturing. Overall, the data presented provides proof of principal that FBS can be replaced with a chemically defined, blood-free virus production media known as OptiVERO® that successfully demonstrates comparable expansion to serum containing media in the iCELLis® bioreactor and is therefore suitable for clinical application.
Materials and Methods
Cells
VERO cells used for cell propagation experiments were originally obtained from ATCC (CCL-81; Manassas, VA). VERO cells were routinely cultured in flasks (Corning, Corning, NY) at 37⁰C/5% CO2 in humidified incubators and passaged every 3-5 days.
Reagents
The complete OptiVERO® media was prepared by thawing the frozen protein and media supplement and aseptically adding directly to the base media. EMEM (MediaTech, Manassas, VA) supplemented with FBS (ATCC Manassas, VA) was used as the traditional FBS-containing medium control in cell expansion. Media was supplemented with antibiotics to a 0.1x final for 2D flask expansion and to a 1x final for expansion in the iCELLis®.
VERO seed train flask expansion
Early passage VERO cells maintained in EMEM-10% FBS were extensively washed with DPBS to remove all traces of serum and subsequently removed from the growth surface using 0.05 mL/cm2 TrypLE containing 1 mM EDTA. Equal volume of 0.25% soybean trypsin was added, and cells were collected with the addition of 0.2 mL/cm2 DPBS. Cells were centrifuged at 1000 RPM for 5 minutes and resuspended in a minimal volume of prewarmed OptiVERO®. Cells were seeded in T-75 flasks containing prewarmed EMEM-10% FBS or OptiVERO® SFM at an initial cell density of 20,000 cells/cm2. VERO cells were subcultured every 3-5 days and subsequently expanded until enough flasks were attainable to yield the recommended iCELLis® bioreactor seeding density of 15,000 to 20,000 cells/cm2.
iCELLis® Bioreactor (3D) cell expansion
One day prior to seed, the iCELLis® nano bioreactors were assembled and the pH and dissolved oxygen (DO) probes were calibrated according to Pall’s instructions. Bioreactors and accompanying assemblies were sterilized in an autoclave and allowed to cool. Initial batching and equilibration of media was done by adding 650 mL of cold complete OptiVERO® SFM or EMEM-10% FBS respectively to the bioreactor. The bioreactor was run for at least 4 hours and up to 12 hours with a DO setpoint of 95%, linear feed rate of 2 cm/s, pH setpoint of 7.25 and temperature setpoint of 37°C. On the day of seed, flasks were harvested and a cell count was taken to determine the volume of inoculum required to achieve 15,000 to 20,000 cells/cm2 in the iCELLis®. Cells were seeded by loading the calculated required volume of cell suspension into the transfer assembly and pumping into the bioreactor. The remaining volume of media required to bring the bioreactor to a final 0.18 mL/cm2 working volume was then pumped through the transfer assembly to chase the cell inoculum. Setpoints for temperature and pH were kept at 37°C and 7.25 respectively. Linear feed rate was set to 2 cm/s for OptiVERO® and 1 cm/s for EMEM-10% FBS. DO was set to 95% for OptiVERO® and 50% for EMEM-10% FBS. Bioreactors were sampled daily for glucose consumption, lactate production, offline pH analysis, and offline nuclei counts to monitor culture progress. If required, a recirculation loop was activated 24 hours post bioreactor seed. VERO cells were allowed to grow for up to 5 days in the iCELLis® bioreactors before the run was terminated and final samples were taken.
Results
VERO cells were expanded in the iCELLis® nano bioreactor for 5 days in both EMEM-10% FBS media and OptiVERO® SFM to determine the ability of OptiVERO® to perform in large scale platforms suitable for clinical application. Daily sampling was done to collect offline nuclei counts and population doublings (PDL) were calculated. Cumulative PDL was calculated and averaged over the course of two separate runs, as shown in Figure 1, where EMEM-10% FBS and OptiVERO® exhibited comparable total PDL with 3.32 ± 0.87 and 3.17 ± 0.04, respectively. Similar expansion trends were seen with the T-75 flask controls for both media conditions with 3.47 ± 0.69 total PDL in EMEM-10% FBS and 3.42 ± 0.44 total PDL in OptiVERO®.
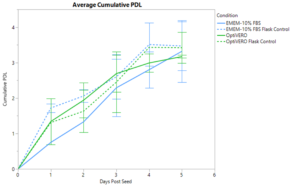
OptiVERO also demonstrated equivalent growth kinetics to EMEM-10% FBS in the iCELLis with a calculated average doubling time of 27.88 ± 7.56 hours in comparison to 34.01 ± 2.24 hours as exhibited by EMEM-10% FBS. Maximum cell densities were calculated to be 1.63e5 ± 0.94e5 nuclei/cm2 in EMEM-10% FBS and 1.24e5 ± 0.19e5 nuclei/cm2 in OptiVERO®.
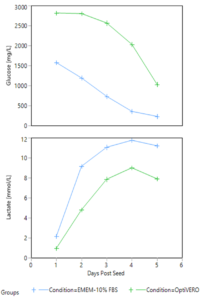
The metabolite profiles of the two media conditions in the iCELLis® were monitored through daily sampling of each media for glucose and lactate concentration. As shown in Figure 2 below, trends were correlated to cell growth with the continuous consumption of glucose and production of lactate over the 5 days of expansion in the bioreactor in both EMEM-10% FBS and OptiVERO® settings. Similarly, pH was sampled offline daily and remained above the setpoint of 7.25 for both EMEM-10% FBS and OptiVERO® throughout the length of the run.
To further demonstrate the compatibility of OptiVERO® in the iCELLis® bioreactors, varying sizes of growth surface areas were tested, ranging from the smallest available nano with 0.53 m2 of surface area up to 2.6 m2 of growth surface area. To normalize the results, nuclei per cm2 were calculated and a summary of the results are shown below in Table 1.
Table 1. Summary of results from 3 separate runs performed to expand VERO cells in OptiVERO SFM in varying sizes of iCELLis bioreactors.
| Run | Bioreactor Size [m2] | Maximum Cell Confluency [nuclei/cm2] | Mean Doubling Time [hours] | Cumulative PDL |
| 1 | 0.53 | 9.89e4 +/- 0.42e4 | 39.96 +/- 9.60 | 2.36 +/- 0.06 |
| 2 | 2.60 | 1.38e5 +/- 0.13e5 | 25.93 +/- 9.91 | 3.20 +/- 0.13 |
| 3 | 0.53 | 1.11e5 +/- 0.08e5 | 31.35 +/- 4.09 | 3.15 +/- 0.11 |
Comparable cell expansion was exhibited across the portfolio of surface area sizes with an average nuclei/cm2 after 5 days post seed of 9.89e4 ± 0.42e4 in the 0.53 m2 bioreactor, 1.38e5 ± 0.13e5 in the 2.6 m2 bioreactor, and 1.11e5 ± 0.08e5 in the 0.53 m2 bioreactor. Doubling time was also calculated and cell growth kinetics were determined to be 39.96 ± 9.60 hours, 25.93 ± 9.91 hours, and 31.35 ± 4.09 hours for Runs 1, 2, and 3 respectively.
Discussion
It is demonstrated here that the novel chemically defined OptiVERO® can support rapid VERO cell proliferation in the iCELLis® bioreactor platform without the need for serum supplementation. VERO cells expanded in OptiVERO® for 5 days in the iCELLis® exhibited cumulative population doublings, maximum cell densities, and doubling times comparable to that observed with EMEM-10% FBS media. Similarly, correlations between glucose consumption and lactate production were indicative of robust cell growth across the two media conditions. As vaccines have become a revolutionary tool in providing protection against severe infectious diseases and other serious conditions, the need for safe, reliable, and efficient biomanufacturing platforms has become all the more apparent, pushing regulatory guidance away from that of serum. Further, FBS comes with many associated risks in lack of reproducibility due to variation of composition as well as reliability of supply [6]. Therefore, OptiVERO® offers an attractive solution to circumvent these poorly defined serum and serum-derived components while still providing the high performance required for vaccine production.
Of equal importance is the platform utilized for manufacturing of viral vaccines to provide the robustness, efficiency, and overall productivity of the system as a whole. Traditional technologies such as stirred tank bioreactors and microcarrier cultures provide low volumetric productivity, high operating costs, and challenging process implications. The innovative Pall iCELLis® bioreactor overcomes many of these manufacturing hurdles by offering a simplified fixed-bed microfiber system that results in increased volumetric productivity, higher process control, and easier adaptability from R&D to clinical manufacturing.
When combined, these two advancements, OptiVERO® chemically defined media and the iCELLis® bioreactor, work synergistically to provide a highly effective solution for viral vector production. These studies presented here exhibit the ability to replace FBS with a chemically defined, serum-free media for robust 3D VERO expansion to ultimately produce the high viral titer required for successful manufacturing of vaccines in development today.
| For additional product or technical information, please contact us at | |
| 2718 Industrial Drive
Junction City, KS 66441 |
Phone: 1.800.916.8311
Email: Info@InVitria.com |
Footnotes
- R. Hegde, “Cell culture-based influenza vaccines: A necessary and indispensable investment for the future,” Hum. Vaccines Immunother., vol. 11, no. 5, pp. 1223–1234, 2015.
- N. Barrett, W. Mundt, O. Kistner, and M. K. Howard, “Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines,” Expert Rev. Vaccines, vol. 8, no. 5, pp. 607–618, May 2009.
- Desmyter, J. L. Melnick, and W. E. Rawls, “Defectiveness of interferon production and of rubella virus interference in a line of African green monkey kidney cells (Vero),” J. Virol., vol. 2, no. 10, pp. 955–961, Oct. 1968.
- A. Govorkova, S. Kodihalli, I. V. Alymova, B. Fanget, and R. G. Webster, “Growth and immunogenicity of influenza viruses cultivated in Vero or MDCK cells and in embryonated chicken eggs,” Dev. Biol. Stand., vol. 98, pp. 39–51; discussion 73-74, 1999.
- “Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products (EMA/410/01 rev.3),” p. 18.
- van der Valk et al., “Fetal Bovine Serum (FBS): Past – Present – Future,” ALTEX, vol. 35, no. 1, pp. 99–118, 2018.
- Alfano, A. Pennybaker, A. Cunningham, P. Halfmann, and C. Huang, “Formulation and production of a blood-free and chemically defined virus production media for VERO cells,” 2019.