- Home
- Why Do Some Biologics Cross the Finish Line—While Others Crash and Burn?
Why Do Some Biologics Cross the Finish Line—While Others Crash and Burn?
Published on 18 March 2025
CMC Planning Could Be the Secret Weapon You’re Missing
Reading time: 4 minutes
Share this article:

Imagine spending years and millions developing a groundbreaking biologic, only to see everything fall apart right at the doorstep of FDA approval. Frustrating, right? Unfortunately, it’s common—and often boils down to one overlooked area: Chemistry, Manufacturing, and Controls (CMC).
So why exactly do promising biologics get derailed by something as seemingly mundane as CMC? Let’s dive in.
Biologics vs. Small Molecules: What’s Really Going On?
Here’s the deal—biologics generally do better than small molecules at every stage of clinical trials, but only if CMC is managed meticulously:
- Preclinical to Phase 1: Biologics succeed 79% of the time, compared to 63% for small molecules.
- Phase 1 to Phase 2: Biologics pull ahead again at 52%, versus 41%.
- Phase 2 to Phase 3: A whopping 67% of biologics move forward, while small molecules trail far behind at 31%.
- Phase 3 to Market Approval: Biologics hold a solid 70% success rate, compared to small molecules’ 58%.
Bottom line: biologics have a clear advantage—but only if you’ve nailed down your CMC strategy.
The Hidden Cost of Late-Stage CMC Failures
Here’s something companies rarely admit: almost half of Biologics License Applications (BLAs) stumble at the finish line due to CMC. Every delay isn’t just a financial hit—it’s also a golden opportunity handed to your competitors.
Real-Life Lessons You Can’t Afford to Ignore
Still skeptical? Let’s talk about some hard-learned industry lessons:
- Eli Lilly’s Liprotamase Debacle: Despite a strong therapeutic profile, regulators questioned its stability data, causing costly delays. Stability isn’t glamorous, but ignoring it can sink your product.
- Chiron’s Fluvirin Vaccine Nightmare: Remember the abrupt disappearance in 2004? Animal-derived ingredients led to contamination worries and FDA suspension. Animal-origin-free alternatives could have prevented this catastrophe.
- Pfizer’s Zyvox Setback: This antibiotic faced major delays in Europe due to inconsistent batch quality. One inconsistency can unravel years of hard work overnight.
Early CMC Planning: Why It’s Non-Negotiable
If you think you can sort out your stability studies or manufacturing scale-ups at the last minute—think again. Proactive CMC strategies mean:
- Starting Stability Studies Early: Kick-off pilot studies months before your NDA submission.
- Scaling Up Manufacturing Early: Optimize your processes well ahead of time. Don’t gamble your biologic’s future on late-stage tweaks.
- Aligning with Regulatory Timelines: If your data isn’t ready when regulators ask, you risk setting off a costly chain reaction of delays.
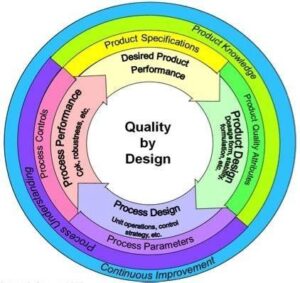
Why You Can’t Afford to Skip Quality by Design (QbD)
Quality by Design isn’t just another buzzword—it’s your best defense against late-stage surprises. Bristol-Myers Squibb learned this the hard way with Abilify Depot. Drug-release inconsistencies caused a year-long delay—something QbD could have prevented.
Different Biologics, Different Risks—Know Yours
Not all biologics are created equal. Here’s how they stack up:
- Vaccines (35%): Clear regulatory paths and solid manufacturing histories help vaccines succeed more often.
- Monoclonal Antibodies & Recombinant Proteins (25%-30%): Established practices provide moderate success.
- Cell & Gene Therapies (15%-20%): Innovative but riskier, facing complex manufacturing and variable patient responses.
Knowing these differences lets you prepare accordingly.

Your Next Move: How to Ensure Your Biologic Doesn’t Falter
The stakes are too high to gamble on your biologic’s future. Here’s exactly what you need to do:
- Take CMC Seriously—Now: Get ahead of stability and scalability challenges early.
- Embrace Animal-Origin-Free Materials: Skip contamination nightmares altogether.
- Implement QbD from Day One: Build quality into your process—don’t try to tack it on later.
- Stay Close to Regulators: Engage frequently and transparently—don’t surprise them with late data dumps.
Don’t become the industry’s next cautionary tale. Take proactive steps today, and let your biologic thrive all the way to market.
At InVitria, we understand the challenges of biologic drug development, especially the risks associated with animal-derived materials. Our animal-origin-free (AOF) raw materials provide greater consistency, enhanced scalability, and improved regulatory compliance—reducing batch-to-batch variability and contamination risks. By integrating chemically defined solutions, biopharma companies can streamline manufacturing, enhance stability, and accelerate approvals.
Ready to secure your biologic’s future? Start strengthening your CMC strategy today. Contact us today to learn how InVitria’s solutions can support your success.
Footnotes
References
- Food and Drug Administration (FDA). (2023). Common deficiencies in biologics license applications. Retrieved from https://www.fda.gov/files/drugs/published/Common-Deficiencies-in-Container-Label-and-Carton-Labeling-for-Biological-Products.pdf
- InVitria. (2024). Benefits of animal-free raw materials in biologic manufacturing. Retrieved from https://invitria.com/resources/regulatory-bodies-shift-animal-origin-free-solutions/
- PQRI/FDA. (2021). Key challenges in biologics manufacturing and regulatory oversight. Retrieved from https://www.pqri.org
- Wong, C. H., Siah, K. W., & Lo, A. W. (2019). Estimation of clinical trial success rates and related parameters. Biostatistics, 20(2), 273-286. https://doi.org/10.1093/biostatistics/kxx069
- PubMed. (2024). Trends in Biologic Drug Approvals from 2009 to 2023. Retrieved from https://pubmed.ncbi.nlm.nih.gov/39585667
- PMC. (2024). Biologic Approvals in 2023. Retrieved from https://pmc.ncbi.nlm.nih.gov/articles/PMC10856271
- Bristol-Myers Squibb (BMS). (2013). Abilify Depot delayed due to manufacturing concerns. Retrieved from https://www.bms.com
- European Medicines Agency (EMA). (2001). Manufacturing inconsistencies delay Zyvox approval. Retrieved from https://www.ema.europa.eu
- Food and Drug Administration (FDA). (2011). Regulatory hurdles for Liprotamase. Retrieved from https://www.fda.gov
- Food and Drug Administration (FDA). (2004). Chiron Corporation’s Fluvirin vaccine contamination concerns. Retrieved from https://www.fda.gov
- Food and Drug Administration (FDA). (2018). Amgen’s Erenumab (Aimovig) faces delays due to sterility assurance issues. Retrieved from https://www.fda.gov
- Rajora, A., & Chhabra, G. (2021). Quality by Design Approach: Regulatory Need, Current, and Future Perspective. ResearchGate. Retrieved from https://www.researchgate.net/figure/Elements-of-quality-by-design-10_fig1_352791050
- Syner-G BioPharma Group. (2024). Understanding large molecule drug development: From biologics to market. Retrieved from https://synergbiopharma.com/understanding-large-molecule-drug-development-from-biologics-to-market/
- CA Academy. (n.d.). FDA Q&As on pathogenic contamination of animal-derived drug ingredients. Retrieved from https://www.gmp-compliance.org/gmp-news/fda-q-as-on-pathogenic-contamination-of-animal-derived-drug-ingredients
Legal Notice
Copyright© 2025 InVitria, Inc. All rights reserved.
Reproduction or distribution of any InVitria materials, in whole or in part, is prohibited without prior written consent. All logos, names, designs, and marks displayed, including InVitria®, and the InVitria® brand design, are trademarks or service marks owned or licensed by InVitria, Inc., Kansas, USA, unless otherwise noted. For details on InVitria’s registered intellectual property, patents, and additional terms and conditions, please visit www.InVitria.com/terms.