Optibumin® 25 – Recombinant human albumin
High-Purity Recombinant Albumin in Closed-System Bags for Advanced Biomanufacturing and Therapeutics
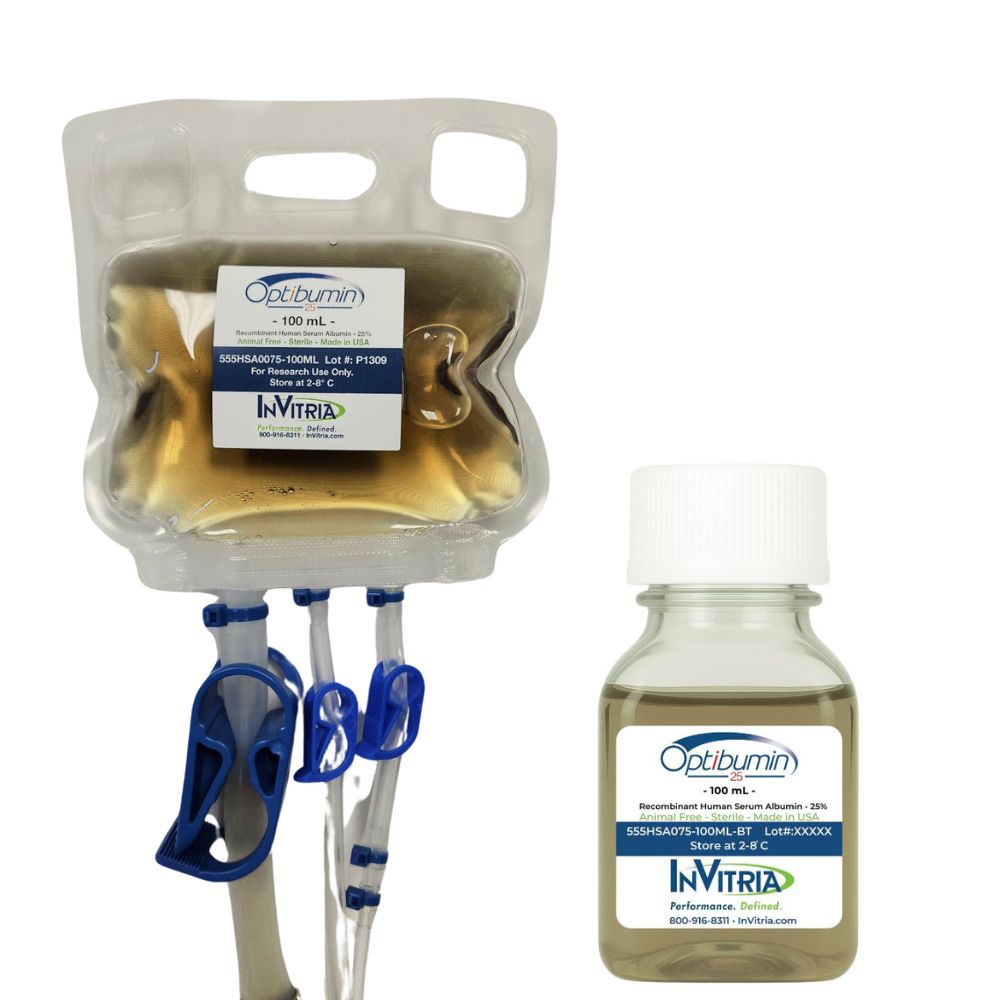

Product Name: Optibumin® 25 – Recombinant human albumin
Product Number: 555HSA0075
Product Form: 25% w/v Liquid
Name | Price | Qty | |
|---|---|---|---|
$785.00
|
Max:
Min: 1
Step: 1
|
||
$785.00
|
Max:
Min: 1
Step: 1
|
Product Description
Optibumin® 25 is a high-purity, recombinant human serum albumin (rHSA) at 25% concentration, designed to replace plasma-derived HSA in cryopreservation, cell therapy, and biomanufacturing applications. As the first recombinant, animal-origin-free 25% HSA solution available in a ready-to-use liquid format, Optibumin 25 eliminates the risks associated with blood-derived albumin, ensuring batch-to-batch consistency, pathogen safety, and regulatory compliance.
Free from stabilizers and preservatives, Optibumin 25 provides a chemically defined, scalable, and sustainable solution for industries requiring superior protein quality in critical workflows.
Advantages
- Superior Purity and Performance
- Highest purity albumin available – free from stabilizers and preservatives.
- Low lipid content and high mercaptoalbumin (Cys34 free thiol) – essential for drug formulation, viral vector stabilization, and cryopreservation applications.
- Lowest product-related high molecular weight impurities – ensuring superior consistency and bioactivity.
- Animal-Origin-Free Assurance
- 100% chemically defined and animal-origin-free (AOF) – eliminates regulatory risks associated with blood-derived HSA.
- Manufactured using a non-animal, non-human host system, ensuring purity and reproducibility.
- Raw materials, manufacturing equipment, and packaging are fully animal-free, aligning with regulatory and ethical standards.
- Manufactured in the United States
- Produced in a tertiary-level, ISO 9001:2015 certified, animal-origin-free facility in the USA.
- Complete supply and manufacturing chain is owned and operated by InVitria, ensuring unmatched traceability and quality control.
- Consistent global supply, free from plasma donation limitations.
- Ready-to-Use Format
- Supplied as a sterile 25% liquid formulation in 100 mL closed-system friendly bags, eliminating the need for reconstitution.
- Seamlessly integrates into closed-system biomanufacturing workflows, reducing contamination risks and streamlining processing.
- Long-term stability and scalability for high-volume manufacturing needs.
Applications
- Cryopreservation & Cell Therapy
- Enables up to 40% DMSO reduction, improving cell viability and patient safety.
- Enhances T cell recovery and expansion, maintaining critical CAR-T memory phenotypes (Tscm, Tcm) post-thaw.
- Provides batch-to-batch consistency, ensuring reproducibility in cell therapy manufacturing.
- Gene Therapy & Vaccine Development
- Stabilizes viral vectors, supporting AAV, LV, and vaccine production.
- Prevents aggregation and loss of biological activity during long-term storage.
- Stem Cell Expansion & Regenerative Medicine
- Supports iPSC, MSC, and ESC culture, providing a chemically defined alternative to serum-derived albumin.
- Enhances cell growth and differentiation, eliminating concerns about plasma variability.
- Biopharmaceutical Formulations & Drug Conjugation
- High mercaptoalbumin (Cys34 free thiol) allows for superior small-molecule solubilization and drug conjugation.
- Ensures greater stability in recombinant protein formulations.
- In Vitro Fertilization (IVF) & Assisted Reproduction
- Ultra-low lipid content improves embryo culture conditions and developmental outcomes.
- Eliminates pathogen concerns associated with blood-derived albumin.
Technical Specifications
- Formulation: Sterile, ready-to-use 25% liquid solution
- Packaging: 100 mL closed-system friendly bags
- Manufacturing: Produced in a state-of-the-art, ISO 9001:2015-certified facility in the USA
- Quality Assurance:
- Fully recombinant and animal-origin-free production system
- Stringent quality control processes ensure high batch-to-batch consistency
- Regulatory Compliance & Safety:
- cGMP-compliant manufacturing
- Plasma-free, pathogen-free, and chemically defined
- Aligned with regulatory expectations for closed-system biomanufacturing
Why Choose Optibumin 25?
Optibumin 25 sets a new standard for high-purity albumin solutions, offering unparalleled consistency, purity, and regulatory compliance compared to plasma-derived HSA. Whether for cryopreservation, cell therapy, vaccine production, or biopharmaceutical manufacturing, Optibumin 25 provides the safest, most scalable, and highest-performing alternative on the market.
See the Terms page for more information about the trademarks and patents covering this product.
Feedback
You must be logged in to post a review.
Reviews
There are no reviews yet.