- Home
- Emergence of Blood-Free Recombinant Human Albumin
Emergence of Blood-Free Recombinant Human Albumin
Published on 20 April 2021
Kaitlyn Vap, Product Applications Associate, InVitria
Emergence of Blood-Free rHSA
Albumin is a naturally occurring protein in animals and humans responsible for numerous cellular functions and is integral to the manufacturing of biopharmaceuticals. Various benefits of albumin inclusion in upstream manufacturing, downstream processing, and final formulation applications will be discussed in detail but can be briefly summarized as:
- Serves as an antioxidant and benefiting stability and preservation
- Limits aggregation in cell culture
- Prevents surface adsorption
- Increases solubility
- Promotes transport and binding
- Protects cells from physical shock and shear
- Enhances API homogeneity
Albumin has historically been sourced from bovine and human donors; however, inconsistency in raw materials ultimately affects patient safety and can cause decreased treatment efficacy. This can look like a shift in cellular phenotype as a result of inconsistent raw materials or poor drug conjugation due to a variance in binding capacity of HSA from lot-to-lot. Additionally, these mammalian sources are not limitless, and the life science industry has already experienced the ramifications of demand outstripping supply in during a global pandemic.1 Blood and blood derived products also can introduce adventitious pathogenic agents into the system that can affect processes, cell culture performance, and downstream drug product purity.
For these reasons, there has been an increase in the development and use of recombinant Human Serum Albumin (rHSA).
Blood-Free Recombinant Albumin
InVitria’s rHSA is derived from a non-mammalian expression system and is Blood-Free and chemically defined. Blood-Free is a term InVitria applies to its products to add clarity and eliminate confusion about the sources and inherent nature of albumin products. Blood-Free simply means the components are not derived from, processed with, or packaged in any serum, animal, human, or other blood source.2 InVitria’s albumin eliminates adventitious agents stemming from mammalian-derived serum and serum proteins, improving patient safety and treatment efficacy. InVitria produces three different rHSA products optimized for different applications:
- Cellastim S® has been optimized for cell culture applications. It is a recombinant human serum albumin manufactured in a completely mammalian-free expression system in a tertiary animal-free production environment. It has been used in countless cell culturing applications and media formulations to provide the qualities of albumin discussed above while eliminating the risks and inefficiencies inherent with animal- and human-derived components.
- Exbumin™ is the first FDA- and EMA-approved recombinant albumin excipient.3 This cGMP grade rHSA has been formulated specifically for production environments up to and including the final formulation of biologics. This supports applications in vaccine development and manufacturing, biological formularies, surgical adhesives, medical devices, and regenerative medicine.
- Optibumin®, the highest-purity albumin available, has a lipid-stripped, high-mercapto profile, optimizing the binding properties of the albumin. The high-purity nature of this rHSA product makes it uniquely suited for biopharmaceutical formularies, surgical adhesives, medical devices, small molecules, diluents, and in vitro fertilization optimization.
Each of these rHSA products is Blood-Free and ready for immediate use. However, choosing the most appropriate product for a given application is as important as avoiding the pitfalls associated with serum-derived albumin. To better understand how to select the proper rHSA, a closer look at albumin’s function and utility within the life sciences and biotechnology applications is necessary.
Functional Elements of Albumin – a Protein with Undefined Potential
Albumin is a 66.5 kDa multifunctional protein synthesized by hepatocytes in the liver. It is composed of three homologous domains each of which contain two subdomains of α-helices as seen in Figure 1. Albumin’s chemical composition, configuration, and three-week half-life make it an efficient carrier and stabilizer protein. Within the body, albumin scavenges radicals (antioxidant), transports a variety of metabolites, supplies the necessary amino acids during malnutrition, and maintains colloid osmotic pressure. Albumin is also leveraged in cell culture media because of the properties it expresses in vivo. In cell culture, albumin serves as an additive for optimized antioxidant capacity, metabolite circulation, and hydrophobic molecule solubilization. In bioreactors, albumin serves to mitigate shear stress normally experienced by the cells.4 Albumin has even been harnessed in drug delivery applications—most notably, Abraxane®.5
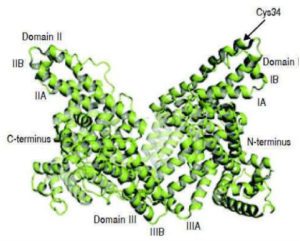
Many of albumin’s properties are mechanistically driven by the thiol group (R-SH) on Cys34 of albumin molecules. In the case of mitigating shear stress, the induced mechanical stressor (i.e., pressure) causes a chemical disruption through the cleavage of intramolecular disulfide bonds. When albumin is exposed to shear stress, the cysteine groups of both intra- and inter-albumin form disulfide bridges causing a coating layer to form through dimerization of two or more albumin molecules. This is referred to as the cavitation effect: rapid changes in pressure to a liquid formulation causing small vapor filled cavities to form where the pressure is low.7 Similar to the cavitation effect, albumin can form the equivalent of micelles in the context of hydrophobic molecule solubilization; hydrophobic molecules reside surrounded by an organized grouping of hydrophilic molecules. This happens via thiol interactions between cysteines on two or more albumin molecules to surround a hydrophobic molecule for solubilization.7 In the case of oxidative stress, the thiol on Cys34 acts as an effective nucleophile and reducing agent by accepting one or two radical electrons, effectively stabilizing the reactive oxygen species (ROS) and reactive nitrogen species (RNS) within the body.8
The circulation of albumin is mediated by cell surface receptors. Glycoproteins (Gp60, Gp30, and Gp18), secreted protein acidic rich in cysteine (SPARC), megalin and cubilin, and FcRn are all cellular receptors that regulate and control the circulation of healthy albumin molecules. Gp60 is responsible for facilitating cellular transcytosis and, along with Gp30 and Gp18, regulate lysosomal degradation, including removing old, damaged, or modified albumin.9 SPARC is an albumin binding protein in the extracellular matrix. It is commonly associated with tissue growth (e.g., tumor proliferation), rendering albumin-bound cancer drugs an efficient method for targeting malignancies.5 Megalin and cubilin work in conjunction by way of receptor mediated endocytosis to prevent renal excretion of albumin. The FcRn receptor regulates the homeostatic content of albumin within the body by mediating cellular catabolism. pH-dependent recycling and transcytosis pathways facilitate the mediation of catabolizing albumin.9 Through the presence of these surface receptors, albumin can effectively transport molecules to targeted locations while maintaining homeostasis.
It is readily apparent that albumin is a critical protein for applications that depend on cell expansion and core function. Thus, albumin is one of the most ubiquitous proteins found in cell culture media that support cell therapy, gene therapy, vaccine development, among other cell culture-dependent biotechnologies. However, its utility extends further than being a component of media.
Beyond Media: Albumin’s Growing Application
Albumin’s ability to bind and transport molecules and APIs makes it an ideal candidate for drug delivery and inclusion in final formulations. Particles for delivery can both covalently and non-covalently bind to albumin for targeted delivery via receptor mediation. Small molecules,7 large molecules,9 viral vectors,10 siRNAs,11 CAR-T,12 etc. can all be conjugated with albumin for optimized cellular delivery, tissue, or organ being targeted in the context of a synthetic or biologic therapeutic. It can also be theorized that albumin’s properties could increase the efficacy of drugs by way of improved half-life and localization to the site of treatment.4 Moreover, in practice it has been proven that albumin increases the efficacy of drugs, such as Abraxane, aldoxorubicin, Levemir®, Victoza®, and ozoralizumab.5 Overall, albumin’s competitive advantages—improved half-life, controlled targeting/delivery, solubilization, etc.—are why it is being considered as an additive in the final formulation of a variety of synthetic and biologic drugs today.
There continues to be increasing interest in replacing serum-derived albumin with rHSA for the reasons previously discussed: performance, supply chain continuity, safety, quality, consistency, regulatory acceptance, and risk mitigation (of contaminants). In fact, regulatory agencies are actively encouraging the use of animal- and human-free sources of these proteins, components, and excipients.13,14 While this may be easier to implement in the initial design of a drug product, Merck’s M-M-R® II vaccine (measles, mumps, and rubella) is an example of reformulating an approved vaccine to replace the albumin with an rHSA. Consistent with the discussion above, Merck sought this change due to “regulatory requirements and limited suppliers of HSA.” 10
It is expected that biopharmaceutical manufacturing and processing will and should continue exploring ways to build rHSA into drug products, which may include reformulation.
Quality by Design & rHSA
While the ideology of Quality by Design (QbD) may have originated in the 1990s, it was not until the FDA incorporated the theory in its 2007 forward-looking guidance that the term began gaining widespread traction within the various life science segments.15 Too often, this philosophy is applied only to the process analytics aspects of a business and loses its focus on the formulary and research elements. However, QbD was developed to ensure that the in vitro performance of processes and drug products themselves translate directly to in vivo performance of those drug products. This begins not only with careful selection of robust methods and APIs, but a holistic approach at each step in the process to mitigate or eliminate the introduction of contamination, variability, and inconsistency. This includes supporting processes upstream through to consideration for the source and quality of excipients contained in the final formulation.
InVitria champions this ideology by providing a safer, high-performance alternative to mammalian-derived albumin by leveraging a highly scalable, non-mammalian expression system to create rHSA. By doing so, risks associated with adventitious agents are eliminated, patient safety and product quality are controlled and maximized, and treatment efficacy is enhanced.
InVitria specializes in working directly with R&D scientists, lab managers, and quality departments to understand the specific needs and match the proper solution to optimize processes and solutions. Regardless of the stage of the therapeutic, from pre-clinical to released product, we encourage you to evaluate the quality of your components, understand the use of albumin in your process, and begin planning the strategies and tactics that will allow you to become Blood-free.
Footnotes
- SJ Stanworth et al. “Effects of the COVID-19 pandemic on supply and use of blood for transfusion.” Lancet Haematol. 2020 Oct;7(10):e756-e764.
- Cell Culture Media & The Emergence of Blood-Free Components. Application Note. https://invitria.com/resources/cell-culture-media-the-emergence-of-blood-freecomponents/.
- Quinn, K. “Novel or Not? Our Inadvertent Journey Filing a Novel Excipient,” IPEC-Americas, webinar, July 22, 2020, https://ipecamericas.org/excipient-learning-lab/webinars/novel-ornot-our-inadvertent-journey-filing-novel-excipient.
- Otagiri, M. and Chuang, V. (2016). ALBUMIN IN MEDICINE: Pathological and clinical applications. Singapore: SPRINGER Verlag, SINGAPOR.
- N. Desai et al. (2009). SPARC Expression Correlates with Tumor Response to AlbuminBound Paclitaxel in Head and Neck Cancer Patients.
- Carvalho, J. and Machado, M. (2018, July 01). New Insights About Albumin and Liver Disease. Retrieved December 10, 2020, from https://www.elsevier.es/en-revista-annalshepatology-16-articulo-new-insights-about-albumin-liver-S1665268119304776 Translational Oncology, 2(2), 59-64. doi:10.1593/tlo.09109.
- Q. Fu et al. (2009). Nanoparticle Albumin – Bound (NAB) Technology is a Promising Method for Anti-Cancer Drug Delivery. Recent Patents on Anti-Cancer Drug Discovery, 4(3), 262-
272. doi:10.2174/157489209789206869. - A. Kawakami et al. (2006). Identification and characterization of oxidized human serum albumin. FEBS Journal, 273(14), 3346-3357. doi:10.1111/j.1742-4658.2006. 05341.x.
- M. T. Larsen et al. (2016). Albumin-based drug delivery: Harnessing nature to cure disease. Molecular and Cellular Therapies, 4(1). doi:10.1186/s40591-016-0048-8.
- R. T. Wiedmann et al., “M-M-R®II manufactured using recombinant human albumin (rHA) and M-M-R®II manufactured using human serum albumin (HSA) exhibit similar safety and immunogenicity profiles when administered as a 2-dose regimen to healthy children,” Vaccine, vol. 33, no. 18, pp. 2132–2140, Apr. 2015, doi: 10.1016/j.vaccine.2015.03.017.
- H. Wen et al. “Encapsulation of RNA by negatively charged human serum albumin via physical interactions.” Sci. China Chem. 60, 130–135 (2017). https://doi.org/10.1007/s11426-016-0094-8.
- Center for Biologics Evaluation and Research. (n.d.). KYMRIAH (tisagenlecleucel). Retrieved April 15, 2021, from https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapyproducts/kymriah-tisagenlecleucel.
- “Note for Guidance on Plasma-Derived Medicinal Products,” The European Agency for the Evaluation of Medicinal Products, Evaluation of Medicines for Human Use, p. 32, 2001.
- C. for B. E. and Research, “Manufacturing Considerations for Licensed and Investigational Cellular and Gene Therapy Products During COVID-19 Public Health Emergency,” U.S. Food
and Drug Administration, Jan. 19, 2021. https://www.fda.gov/regulatoryinformation/search-fda-guidance-documents/manufacturing-considerations-licensed-andinvestigational-cellular-and-gene-therapy-products-during (accessed Mar. 30, 2021). - Pharmaceutical Quality for the 21st Century: A Risk-Based Approach https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm128080.htm.