- Home
- OptiVERO – Expansion of VERO Cells Without Serum in Chemically Defined Cell Culture Media
OptiVERO – Expansion of VERO Cells Without Serum in Chemically Defined Cell Culture Media
Published on 27 February 2019
Annie Cunningham
Combine supplements of OptiVERO Serum Free Media (SFM). Prepare complementary reagents. Wash cells to remove traces of donor serum. Remove cells from growth surface. Wash and collect cells. Centrifuge. Resuspend. Count. Seed in OptiVERO SFM.
Introduction
Vero cells derived from the African green monkey are one of the most common immortalized mammalian cells lines used in laboratory settings. They have many important applications in research [1], including virus production for clinical use. Vero cells have been licensed for production of both live and inactivated viral vaccines.
Microcarrier systems have been shown to increase cellular productivity in optimized, large-scale, cell-virus systems [2]. OptiVERO Serum Free Media is chemically defined and specifically designed and optimized for use with Vero cell lines. OptiVERO SFM contains only synthetic and recombinant components and does not include any animal or human serum-derived ingredients, which may cause variability in the cell-virus system performance and yield.
For the following experiment, we used an adherent Vero Cell line to compare growth in OptiVERO Serum-free media (SFM) and media supplemented with fetal bovine serum (FBS), in both cell-culture flasks and polystyrene microcarriers.
Materials Needed
Growth Media Preparation:
- OptiVERO Components: Base Media, 100X Protein Supplement, 50X Media Supplement
- Gentamicin/amphotericin (Life Technologies R01510)
Cell Passage:
- 75 cm2 tissue culture flask (Falcon 353136)
- 150 cm2 tissue culture flask (Falcon 355001)
- Untreated plastic microcarriers (Corning 3771)
- Soybean Trypsin Inhibitor (Life Technologies 17075-029)
- TrypLE (Life Technologies A1217701)
- 100mM EDTA (Sigma Aldrich E6758)
- Dulbecco’s Phosphate Buffered Solution (dPBS)
- 7 µm nylon cell strainer (Corning 431751)
- 50 mL conical tubes
Optional:
- Minimum Essential Medium (MEM) with Earle’s salts and L-glutamine (Corning 10-010-CV)
- Fetal Bovine Serum (FBS)
- Human Serum (HS)
Equipment:
- 5% CO2 incubator at 37°C, saturating relative humidity
- Class A2 biological safety counter
- Centrifuge
- Cell counter or hemocytometer
Protocol
Growth Media Preparation
- To generate 1 liter of complete OptiVERO SFM, thaw one OptiVERO Media Supplement and one OptiVERO Protein Supplement in a 37⁰C water bath. Do not subject the Protein Supplement to multiple freeze-thaw cycles.
- Once the supplements are completely thawed, mix them by gently pipetting up and down. Do not vortex.
- Add 20 mL from the OptiVERO Media Supplement directly to the OptiVERO Base Media.
- Add 20 mL of OptiVERO Protein Supplement directly to the OptiVERO Base Media.
- This medium contains HEPES and glutamine source. If desired, add gentamicin/amphotericin at 0.1 to 0.5x final concentration. Do not add to a 1x final concentration.
- Avoid repetitive heating and cooling of the complete medium by withdrawing and pre-warming only the volume needed in the final culture vessel to be used.
Reagent Preparation
- Dilute TrypLE to 1x in dPBS and add EDTA to 1 mM final concentration (0.05 mL/cm2 volume needed).
- Dilute 50X soybean trypsin inhibitor to 1x in dPBS (0.05 mL/cm2 volume needed).
Culture Adaptation to Serum-Free Medium
- Aliquot 0.2 mL/cm2 OptiVERO SFM into the desired culture vessel and allow the media to equilibrate in the incubator for at least 30 minutes. Prewarm the diluted soybean trypsin inhibitor and 1x TrypLE in a 37˚C water bath.
- Wash VERO cells with 0.5 mL/cm2 dPBS twice to remove all traces of serum.
- Remove cells from the growth surface using 0.05 mL/cm2 TrypLE/EDTA (NOTE: do not expose cells to the TrypLE/EDTA for longer than 6 minutes)
- Tap the side of the vessel to ensure all cells have been removed from the growth surface.
- After cell detachment is observed, add an equal volume of soybean trypsin inhibitor followed by 0.2 mL/cm2 dPBS to dilute out the enzyme. Collect the cells in a 50mL conical tube.
- Wash the surface and collect any remaining cells with 5-10 mL prewarmed media.
- Pellet by centrifugation at 1000 RPM for 5 minutes.
- Resuspend cells in a minimal volume of prewarmed OptiVERO SFM and count.
- Seed cells at an initial cell density of 10,000-20,000 cells/cm2. VERO cells should be sub-cultured every 3 to 5 days.
- At time of subculture, repeat the procedure above.
Cell Growth in Plastic Microcarriers
- The typical surface area/mL for plastic microcarriers (MC) is shown in Table 1. Development runs consisted of 150 mL of media containing 10 cm2/mL MCs for a total of 1500 cm2 spinners.
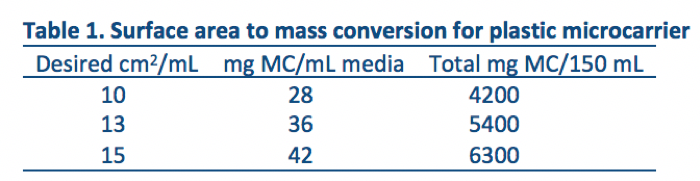
Table 1 - Wash and dry spinner vessel according to house procedures. Autoclave to sterilize.
- Allow the spinner to cool for at least 4 hours prior to starting inoculation process.
- When the microcarriers are ready, add 100 mL of cold OptiVERO SFM immediately followed by MCs to the vessel. Do not allow OptiVERO SFM to warm in the spinner without microcarriers as excessive adsorption of media components to the glass will affect cell adhesion to the MCs.
- NOTE: MCs add ~7-20 mL of volume depending on the amount of MCs and residual volume from the MC washes. Therefore, adjust the amount of media used to transfer the MCs into the vessel. Total volume of spinner after MC addition should be approximately 135 mL.
- Wash tube containing the MCs with an additional volume of OptiVERO SFM to be sure to transfer all MCs.
- Transfer the spinner to the incubator and begin stirring the MCs at 40-50 RPM.
- Allow the spinner to equilibrate for at least 2 hours prior to the inoculation of the VERO cells.
Cell Inoculum Preparation
- VERO cells at 70-80% confluence in 2, T-150 flasks are generally adequate to seed 1 spinner at an initial cell density of 10,000 cells/cm2.
- Wash and trypsinize cells according to the subculture section of this protocol.
- Pellet and resuspend cells in 5 mL of prewarmed OptiVERO SFM.
- Prior to seeding cells into the spinner, pass the cell suspension through a 0.7 µm nylon cell strainer in order to obtain a single-cell suspension.
- Count cells and adjust to 1×106 viable cells/mL in a total of 15 mL of OptiVERO SFM.
- Obtain the equilibrated spinner from the incubator.
- While the spinner is stirring at 40 RPM in the hood, slowly add the 15 mL of cell suspension.
- Place the spinner back in the incubator and do not disturb the spinner for at least 4 hrs.
Monitoring the MicroCarrier Run
- Starting at Day 2, spinners should be monitored every day for culture glucose levels and pH.
- Culture glucose should be maintained at 2 g/L final by the addition of a 45% glucose solution.
- Culture pH should be maintained between 6.9 and 7.2 with the addition of 7.5% sodium bicarbonate solution.
- If desired, culture samples can be obtained at this time to monitor cell growth and quantified by method of choice.
Results and Discussion:
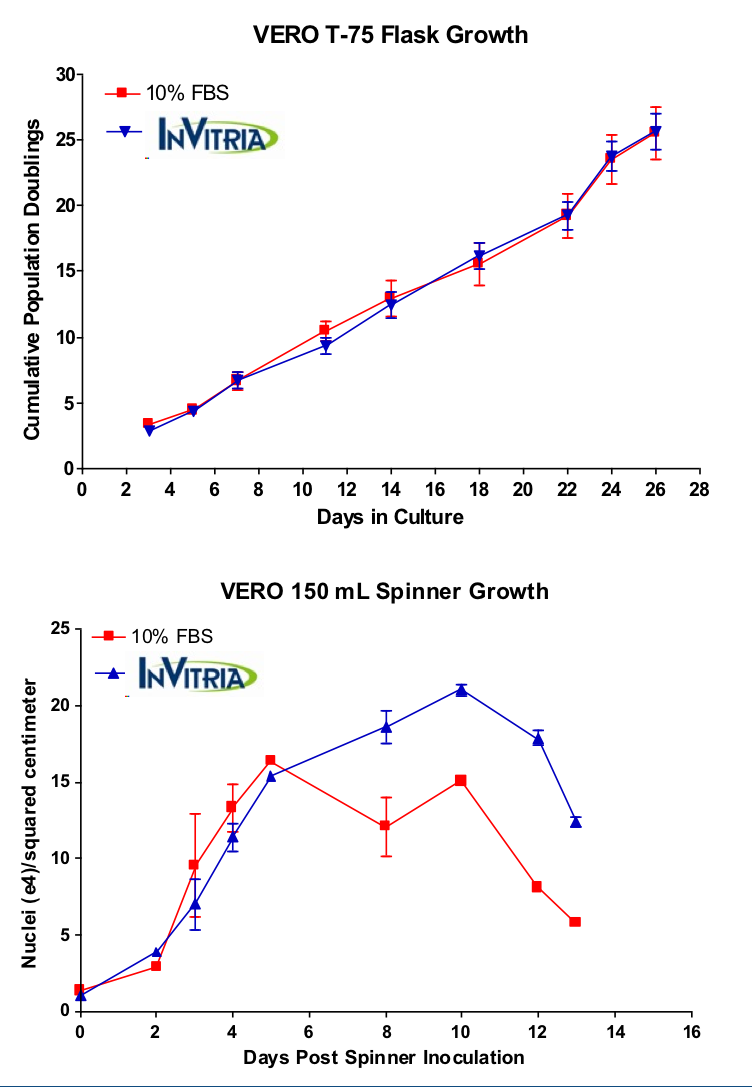
In contrast to many therapeutic medicines, vaccines are specifically administered to healthy people to help prevent illness. Therefore, the need for reliable, safe and high-performing cell culture systems and media, free of contamination from adventitious pathogens like viruses and prions, is critical. Furthermore, cell-based biomanufacturing of vaccines is media-intensive and may require quantities of serum that would be impractical and cost-prohibitive to source. Our data shows cumulative population doublings of cells in OptiVERO SFM were measured in culture over 28 days in T-75 flasks. Expansion of Vero cells grown in OptiVERO SFM was comparable to that of cells in 10% Fetal Bovine Serum (FBS). Furthermore, nuclei per square centimeter were measured in Vero cells grown in 150 mL spinner flasks. Growth of Vero cells in OptiVERO SFM outperformed Vero cells in 10% FBS. The increased cell performance in spinner flasks shows the benefits of using a chemically defined media when expanding Vero cell lines to support virus production. Therefore, development of chemically defined, serum-free media to support virus production will be essential to make this technique a reality.
The following content is gated. Please, subscribe to open access to it.
Footnotes
- Ammerman NC, Beier-Sexton M, Azad AF. Growth and maintenance of Vero cell lines. Curr Protoc Microbiol. 2008;Appendix 4:Appendix 4E.
- Giard DJ, Thilly WG, Wang DI, Levine DW. Virus production with a newly developed microcarrier system. Appl Environ Microbiol. 1977;34(6):668-672.