- Home
- Optibumin vs Traditional Albumin: Comparing Performance in Cell Wash Applications
Optibumin vs Traditional Albumin: Comparing Performance in Cell Wash Applications
Published on 29 October 2024
How Optibumin Outperforms Traditional Albumin in Cell Therapy: Boosting Cell Retention, Viability, and Safety with Scalability, Recombinant Biomanufacturing Solutions
Reading time: 5 minutes
Share this article:

In this article, we will explore the findings from recent experiments comparing Optibumin and traditional serum-derived albumin in cell wash applications. The study, led by Dr. Stathos and detailed in our latest application note, demonstrates how Optibumin matches or outperforms HSA in maintaining cell count and viability, making it a compelling option for biomanufacturers looking to scale their cell therapy processes with confidence.
Why Albumin Matters in Cell Washing
Cell washing is an essential step in the production of cell therapies, helping to remove residual media components and contaminants before final formulation or cryopreservation. Albumin plays a key role during this process, offering several benefits:
- Protection from Oxidation and Mechanical Stress: Albumin stabilizes cells, preventing damage during handling.
- Binding and Removal of Unwanted Components: It helps clear out unwanted substances, ensuring cleaner, purer cell products.
- Cryoprotective Properties: Albumin’s unique properties also make it valuable in cryopreservation, protecting cells from damage during freezing and thawing.
The Limitations of Traditional Serum-Derived Albumin
While HSA has been a staple in cell therapy processes, it presents several challenges:
- Safety Concerns: Serum-derived albumin can carry risks of contamination and variability due to its human donor origin.
- Batch Variability: Donor heterogeneity leads to inconsistent quality between batches can lead to process inefficiencies and quality control issues.
- Xeno-derived Contamination: Traditional HSA comes from human or animal sources, which increases the risk of introducing xeno-derived pathogens or components into cell cultures.
- Supply Chain Issues: As a human-derived product, HSA is subject to supply fluctuations, recalls and shortages, which can disrupt production schedules.
- Scalability Challenges: Sourcing consistent, high-quality HSA can be difficult at scale, creating bottlenecks in manufacturing processes.
- Pandemic-Related Supply Risks: The COVID-19 pandemic highlighted significant vulnerabilities in the supply of blood-derived products, including HSA. The unknown risk of pathogen carryover, coupled with reduced blood donations during the pandemic, led to supply disruptions and raised concerns about the safety and availability of traditional albumin.
- Quality Burden of Screening: Incoming batches of serum albumin require extensive screening for viruses and other contaminants, adding significant costs, resources, and time to ensure the product is safe and free from pathogens. This burden increases as production scales, straining both budget and operational bandwidth.
Optibumin: A Reliable Animal-free, Plant-Based and Scalable Solution
Optibumin is a high-performance, chemically defined recombinant human albumin produced using non-mammalian expression systems, ensuring a consistent, high-quality and scalable product. Unlike traditional HSA, Optibumin is not dependent on animal-derived sources, making it a more reliable choice, especially during periods of global disruption like the COVID-19 pandemic. Key benefits of Optibumin include:
- Animal-Free and Xeno-Free Origin: Optibumin eliminates the risk of contamination from animal-derived components, making it an ideal solution for biomanufacturers focused on safety, compliance and scalability. Its plant-based production aligns with sustainability and innovation.
- Chemically Defined and Consistent Quality: Manufactured under controlled conditions, Optibumin ensures batch-to-batch consistency, even as production scales up. This reproducibility is critical in reducing variability and improving process efficiency.
- Scalability: Designed for large-scale biomanufacturing processes, Optibumin supports streamlined integration, ensuring availability and consistent performance across production volumes.
- Safe and Reliable: Free from donor variability and xeno-derived risks, and the uncertainties of blood supply, ensuring predictable performance in biomanufacturing processes, even during global crises.
- Serum-free: Optibumin’s formulation does not rely on serum, removing the variability and contamination risks associated with serum-based products, while supporting higher product consistency.
Experimental Findings: Optibumin vs. Traditional HSA
Recent experiments, as described in the application note “Maintaining T Cell Count, Viability, and Health During Cell Washing Using Optibumin,” compared the performance of Optibumin and HSA in maintaining T cell count and viability during the cell washing process. The study focused on key metrics, including cell retention and viability across different conditions, using Plasma-Lyte and PBS buffer systems.
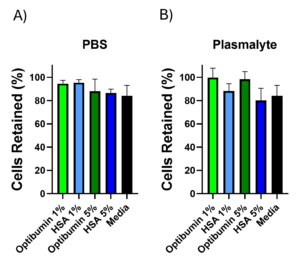
Cell RetentionResults: Optibumin demonstrated superior or comparable performance to HSA in maintaining cell count after washing. Specifically, when T cells were washed using Optibumin in a Plasma-Lyte buffer, cell retention was consistently higher than that seen with HSA. The application note highlights that Optibumin achieved over 85% cell retention across multiple concentrations, outperforming HSA, which showed variability and lower retention rates under the same conditions.
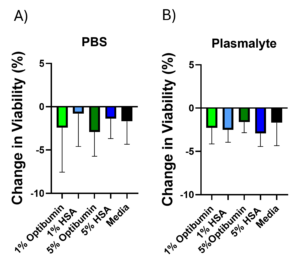
Results: The study also showed that Optibumin maintained high cell viability, with results comparable to those achieved using traditional HSA. For instance, under Plasma-Lyte conditions, Optibumin preserved cell viability above 95%, even when cells were subjected to extended wash durations. The data also revealed minimal changes in viability, ensuring cells remained healthy and viable throughout the washing process.
Why Optibumin is the Superior Choice for Cell Washing
Biomanufacturers need reliable, high-quality components to ensure the success of their products. Optibumin offers:
- High-Performance, Animal-Free and Plant-Based: A combination that ensures safety, sustainability, and regulatory compliance, appealing to a broader market.
- Chemically Defined Consistency Across Scales: Reliable performance across batches, ensuring process predictability whether in small-scale labs or full-scale manufacturing.
- Regulatory Compliance: The animal-free, xeno-free nature of Optibumin makes it ideal for manufacturers navigating stringent regulatory environments.
- Scalability: Unlike traditional serum-derived albumin, Optibumin is designed for efficient scale-up, ensuring consistent supply and quality, supporting smooth incorporation into large-scale production workflows.
- Flexibility: Suitable for various cell washing applications, supporting seamless integration into existing workflows.
- Pandemic-Resilient Supply: Optibumin’s production is not dependent on blood donations, making it a more resilient choice during times of crisis. During the COVID-19 pandemic, blood-derived product supplies faced significant disruptions due to reduced donations and concerns over pathogen carryover. Optibumin helps mitigate these risks by providing a stable and safe alternative.
The experimental findings detailed in our application note underscore Optibumin’s ability to match or exceed the performance of traditional HSA, making it a compelling alternative for biomanufacturers aiming to optimize their cell therapy processes, reduce variability, enhance product quality and scale up production effectively.
Regulatory Compliance: Meeting Global Standards With Optibumin
Regulatory compliance is essential in biomanufacturing, ensuring the safety, quality, and consistency of products. Optibumin is engineered to help manufacturers meet these stringent standards by offering a high-quality, animal-free, plant-based solution that aligns with key regulatory requirements. Designed to adhere to U.S. FDA regulations under 21 CFR Part 210 & 211 (GMP) as an excipient, Optibumin ensures that products are produced to the highest quality standards. Its animal-free formulation also minimizes contamination risks, supporting compliance with additional biologics regulations under 21 CFR Part 600.
Download the App Note: Maintaining T Cell Count, Viability, and Health During Cell Washing Using Optibumin
Ready to Make the Switch?
Optibumin is setting new standards in biomanufacturing by offering a high-purity, consistent, recombinant and scalable alternative to traditional serum-derived albumin. Interested in learning more or trying Optibumin in your process? Contact our team today to experience the benefits for yourself.
Ready to scale your cell therapy manufacturing process? Contact us or call 800-916-8311 to learn how InVitria’s animal-free, recombinant solutions can help you achieve the consistency, safety, and scalability necessary for clinical and commercial success.
Featured Products
 Optibumin® – Recombinant Human Albumin
Optibumin® – Recombinant Human Albumin
Optibumin enhances cell viability and growth, making it ideal for biomanufacturing, cell therapy and vaccine production. This high-purity, recombinant, animal-free, plant based, albumin solution features a chemically defined formulation, ensuring consistent quality and safety. Optibumin eliminates risks associated with animal- and human-derived materials, supporting reliable, scalable production processes.
Footnotes
References:
- Stathos, D. (2024). Maintaining T Cell Count, Viability, and Health During Cell Washing Using Optibumin. InVitria. Retrieved from https://invitria.com/resources/optibumin-tcell-washing
- S. Food and Drug Administration (FDA). (2023). Code of Federal Regulations Title 21, Parts 210 & 211: Current Good Manufacturing Practice for Drugs. Retrieved from https://www.fda.gov
Legal Notice
Copyright© 2024 InVitria, Inc. All rights reserved.
Reproduction or distribution of any InVitria materials, in whole or in part, is prohibited without prior written consent. All logos, names, designs, and marks displayed, including InVitria®, and the InVitria® brand design, are trademarks or service marks owned or licensed by InVitria, Inc., Kansas, USA, unless otherwise noted. For details on InVitria’s registered intellectual property, patents, and additional terms and conditions, please visit www.InVitria.com/terms.